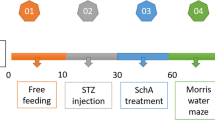
Abstract
Diabetes-associated cognitive dysfunction is linked to chronic hyperglycemia, oxidative stress, inflammation, cholinergic dysfunction, and neuronal degeneration. We investigated the antidiabetic and neuroprotective activity of a mixture of Sclerocarya birrea, Nauclea latifolia, and Piper longum (SNP) in type 2 diabetic (T2D) rat model-induced memory impairment. Fructose (10%) and streptozotocin (35 mg/kg) were used to induce T2D in male Wistar rats. Diabetic animals received distilled water, metformin (200 mg/kg), or SNP mixture (75, 150, or 300 mg/kg). HPLC–MS profiling of the mixture was performed. Behavioral testing was conducted using the Y-maze, NORT, and Morris water mazes to assess learning and memory. Biochemical markers were evaluated, including carbohydrate metabolism, oxidative/nitrative stress, pro-inflammatory markers, and acetylcholinesterase activity. Histopathological examination of the pancreas and hippocampus was also performed. Fructose/STZ administration resulted in T2D, impaired short- and long-term memory, significantly increased oxidative/nitrative stress, pro-inflammatory cytokine levels, acetylcholinesterase activity (AChE), hippocampal neuronal loss and degeneration in CA1 and CA3 subfields, and neuronal vacuolation in DG. SNP mixture at 150 and 300 mg/kg significantly improved blood glucose and memory function in diabetic rats. The mixture reduced oxidative/nitrative stress and increased endogenous antioxidant levels. It also reduced serum IL-1β, INF-γ and TNF-α levels and ameliorated AChE activity. Histologically, SNP protected hippocampus neurons against T2D-induced neuronal necrosis and degeneration. We conclude that the aqueous extract of SNP mixture has antidiabetic and neuroprotective activities thanks to active metabolites identified in the plant mixture, which consequently normalized blood glucose, protected hippocampus neurons, and improved memory function in diabetic rats.
Graphical abstract














Similar content being viewed by others
Data availability
The figures and tables supporting the results of this study are included in the article and the original datasets are available from the first author or corresponding author upon request.
Code availability
Not applicable.
Abbreviations
- SNP:
-
Sclerocarya birrea-Nauclea latifolia-Piper longum
- CNS:
-
Central nervous system
- T2D:
-
Type 2 diabetes
- STZ:
-
Streptozotocin
- BGL:
-
Blood glucose level
- MWM:
-
Morris water maze
- NORT:
-
Novel object recognition test
- AChE:
-
Acetylcholinesterase
- ROS:
-
Reactive oxygen species
- MDA:
-
Malondialdehyde
- GSH:
-
Reduced glutathione
- IL-1β:
-
Interleukin-1β
- TNF-α:
-
Tumor necrosis factor-α
- INF-γ:
-
Interferon-γ
- HPLC-MS:
-
High performance liquid chromatography-mass spectrometry
- ELISA:
-
Enzyme-linked immunosorbent assay
- i.v.:
-
Intravenously
- p.o. :
-
Per os
- HOMA:
-
Homeostasis model assessment
- CV:
-
Cresyl violet
- H-E:
-
Hematoxylin-eosin
References
Akash MSH, Rehman K, Liaqat A (2018) Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem 119(1):105–110. https://doi.org/10.1002/jcb.26174
Alarcon-Gil J, Sierra-Magro A, Morales-Garcia JA, Sanz-SanCristobal M, Alonso-Gil S, Cortes-Canteli M, Niso-Santano M, Martínez-Chacón G, Fuentes JM, Santos A, Perez-Castillo A (2022) Neuroprotective and anti-inflammatory effects of linoleic acid in models of Parkinson's disease: the implication of lipid droplets and lipophagy. Cells 11(15). https://doi.org/10.3390/cells11152297
Amouzoun L-L, Agbonon A, Eklu-Gadegbeku K, Aklikokou K, Gbéassor M (2008) Activités antipyrétique et anti-inflammatoire d’extraits hydro-alcooliques des racines et feuilles de Nauclea latifolia Smith (Rubiaceae) chez le rat Wistar. Phytothérapie 6(4):228–231
Arena A, Zimmer TS, van Scheppingen J, Korotkov A, Anink JJ, Mühlebner A, Jansen FE, van Hecke W, Spliet WG, van Rijen PC (2019) Oxidative stress and inflammation in a spectrum of epileptogenic cortical malformations: molecular insights into their interdependence. Brain Pathol 29(3):351–365
Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, Hughes TM, Craft S, Freedman BI, Bowden DW, Vinik AI, Casellini CM (2017) Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab 102(12):4343–4410. https://doi.org/10.1210/jc.2017-01922
Bhutada P, Mundhada Y, Bansod K, Bhutada C, Tawari S, Dixit P, Mundhada D (2010) Ameliorative effect of quercetin on memory dysfunction in streptozotocin-induced diabetic rats. Neurobiol Learn Mem 94(3):293–302. https://doi.org/10.1016/j.nlm.2010.06.008
Biessels GJ, Kamal A, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH (1998) Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res 800(1):125–135. https://doi.org/10.1016/s0006-8993(98)00510-1
Butterfield DA, Di Domenico F, Barone E (2014) Elevated risk of type 2 diabetes for development of Alzheimer disease: a key role for oxidative stress in brain. Biochim Biophys Acta 1842(9):1693–1706. https://doi.org/10.1016/j.bbadis.2014.06.010
Castro MFV, Stefanello N, Assmann CE, Baldissarelli J, Bagatini MD, da Silva AD, da Costa P, Borba L, da Cruz IBM, Morsch VM, Schetinger MRC (2021) Modulatory effects of caffeic acid on purinergic and cholinergic systems and oxi-inflammatory parameters of streptozotocin-induced diabetic rats. Life Sci 277:119421. https://doi.org/10.1016/j.lfs.2021.119421
Cerasuolo J, Izzo A (2017) Persistent impairment in working memory following severe hyperglycemia in newly diagnosed type 2 diabetes. Endocrinol Diabetes Metab Case Rep 2017. https://doi.org/10.1530/edm-17-0101
Chukwuma CI, Matsabisa MG, Ibrahim MA, Erukainure OL, Chabalala MH, Islam MS (2019) Medicinal plants with concomitant anti-diabetic and anti-hypertensive effects as potential sources of dual acting therapies against diabetes and hypertension: a review. J Ethnopharmacol 235:329–360. https://doi.org/10.1016/j.jep.2019.02.024
Cozachenco D, Selles MC, Ribeiro FC (2019) Interferon-γ as a potential link between diabetes mellitus and dementia. J Neurosci 39(24):4632–4635
Dimo T, Rakotonirina SV, Tan PV, Azay J, Dongo E, Kamtchouing P, Cros G (2007) Effect of Sclerocarya birrea (Anacardiaceae) stem bark methylene chloride/methanol extract on streptozotocin-diabetic rats. J Ethnopharmacol 110(3):434–438. https://doi.org/10.1016/j.jep.2006.10.020
Driscoll I, Shumaker SA, Snively BM, Margolis KL, Manson JE, Vitolins MZ, Rossom RC, Espeland MA (2016) Relationships between caffeine intake and risk for probable dementia or global cognitive impairment: the Women’s Health Initiative Memory Study. J Gerontol Ser A 71(12):1596–1602
Duff M, Demidova O, Blackburn S, Shubrook J (2015) Cutaneous manifestations of diabetes mellitus. Clin Diabetes 33(1):40–48
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Estévez M (2011) Protein carbonyls in meat systems: a review. Meat Sci 89(3):259–279. https://doi.org/10.1016/j.meatsci.2011.04.025
Fukui K, Onodera K, Shinkai T, Suzuki S, Urano S (2001) Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Ann N Y Acad Sci 928(1):168–175
Gheibi S, Kashfi K, Ghasemi A (2017) A practical guide for induction of type-2 diabetes in rat: incorporating a high-fat diet and streptozotocin. Biomed Pharmacother 95:605–613
Gidado A, Ameh DA, Atawodi SE, Ibrahim S (2008) Hypoglycaemic activity of Nauclea latifolia Sm. (Rubiaceae) in experimental animals. Afr J Tradit Complement Altern Med 5(2):201–208. https://doi.org/10.4314/ajtcam.v5i2.31274
Hacioglu C, Kar F, Kara Y, Yucel E, Donmez DB, Sentürk H, Kanbak G (2021) Comparative effects of metformin and Cistus laurifolius L. extract in streptozotocin-induced diabetic rat model: oxidative, inflammatory, apoptotic, and histopathological analyzes. Environ Sci Pollut Res 28(41):57888–57901
Hansen DV, Hanson JE, Sheng M (2018) Microglia in Alzheimer’s disease. J Cell Biol 217(2):459–472
Harandi S, Golchin L, Ansari M, Moradi A, Shabani M, Sheibani V (2015) Antiamnesic effects of walnuts consumption on scopolamine-induced memory impairments in rats. Basic Clin Neurosci 6(2):91–99
Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD (2009) Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiat 66(5):415–422
Herder C, Brunner EJ, Rathmann W, Strassburger K, Tabák AG, Schloot NC, Witte DR (2009) Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the Whitehall II study. Diabetes Care 32(3):421–423
Jiao Z, Zhang W, Chen C, Zhu X, Chen X, Zhou M, Peng G, Liu H, Qiu J, Lin Y (2018) Gene dysfunction mediates immune response to dopaminergic degeneration in Parkinson’s disease. ACS Chem Neurosci 10(2):803–811
Khan H, Ullah H, Aschner M, Cheang WS, Akkol EK (2019) Neuroprotective effects of quercetin in Alzheimer's disease. Biomolecules 10(1).https://doi.org/10.3390/biom10010059
Kilari EK, Rao LSN, Sreemanthula S, Kola PK (2015) Anti-stress and nootropic activity of aqueous extract of Piper longum fruit, estimated by noninvasive biomarkers and Y-maze test in rodents. Environ Exp Biol 13:25–31
Kim YG, Baltabekova AZ, Zhiyenbay EE, Aksambayeva AS, Shagyrova ZS, Khannanov R, Ramanculov EM, Shustov AV (2017) Recombinant Vaccinia virus-coded interferon inhibitor B18R: Expression, refolding and a use in a mammalian expression system with a RNA-vector. PLoS ONE 12(12):e0189308
Kothari V, Galdo JA, Mathews ST (2016) Hypoglycemic agents and potential anti-inflammatory activity. J Inflamm Res 9:27
Kouémou NE, Taiwe GS, Moto FCO, Pale S, Ngoupaye GT, Njapdounke JSK, Nkantchoua GCN, Pahaye DB, Bum EN (2017) Nootropic and neuroprotective effects of dichrocephala integrifolia on scopolamine mouse model of Alzheimer’s disease. Front Pharmacol 8:847. https://doi.org/10.3389/fphar.2017.00847
Kraeuter AK, Guest PC, Sarnyai Z (2019) The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol 1916:105–111. https://doi.org/10.1007/978-1-4939-8994-2_10
Kumar S, Sharma S, Suman J (2011) In vivo anti-hyperglycemic and antioxidant potential of Piper longum fruit. J Pharm Res 4:471–474
Lang BT, Yan Y, Dempsey RJ, Vemuganti R (2009) Impaired neurogenesis in adult type-2 diabetic rats. Brain Res 1258:25–33. https://doi.org/10.1016/j.brainres.2008.12.026
Li XH, Xin X, Wang Y, Wu JZ, Jin ZD, Ma LN, Nie CJ, Xiao X, Hu Y, Jin MW (2013) Pentamethylquercetin protects against diabetes-related cognitive deficits in diabetic Goto-Kakizaki rats. J Alzheimers Dis 34(3):755–767. https://doi.org/10.3233/jad-122017
Liu KF, Niu CS, Tsai JC, Yang CL, Peng WH, Niu HS (2022) Comparison of area under the curve in various models of diabetic rats receiving chronic medication. Arch Med Sci 18(4):1078–1087. https://doi.org/10.5114/aoms.2019.91471
Lotfy M, Adeghate J, Kalasz H, Singh J, Adeghate E (2017) Chronic complications of diabetes mellitus: a mini review. Curr Diabetes Rev 13(1):3–10. https://doi.org/10.2174/1573399812666151016101622
Lum PT, Sekar M, Gan SH, Pandy V, Bonam SR (2021) Protective effect of mangiferin on memory impairment: a systematic review. Saudi J Biol Sci 28(1):917–927. https://doi.org/10.1016/j.sjbs.2020.11.037
Mathiasen JR, DiCamillo A (2010) Novel object recognition in the rat: a facile assay for cognitive function. Curr Protoc Pharmacol. https://doi.org/10.1002/0471141755.ph0559s49. (Chapter 5, Unit 5.59)
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. https://doi.org/10.1007/bf00280883
McManus RM, Higgins SC, Mills KH, Lynch MA (2014) Respiratory infection promotes T cell infiltration and amyloid-β deposition in APP/PS1 mice. Neurobiol Aging 35(1):109–121
Mehta BK, Banerjee S (2017) Characterization of cognitive impairment in type 2 diabetic rats. Indian J Pharm Sci 79(5):785–793
Mendiola AS, Cardona AE (2018) The IL-1β phenomena in neuroinflammatory diseases. J Neural Transm (Vienna) 125(5):781–795. https://doi.org/10.1007/s00702-017-1732-9
Miranda CA, Schönholzer TE, Klöppel E, Sinzato YK, Volpato GT, Damasceno DC, Campos KE (2019) Repercussions of low fructose-drinking water in male rats. An Acad Bras Cienc 91(1):e20170705. https://doi.org/10.1590/0001-3765201920170705
Moheet A, Mangia S, Seaquist ER (2015) Impact of diabetes on cognitive function and brain structure. Ann N Y Acad Sci 1353(1):60–71
Nabi SA, Kasetti RB, Sirasanagandla S, Tilak TK, Kumar MV, Rao CA (2013) Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced diabetic rats. BMC Complement Altern Med 13:37. https://doi.org/10.1186/1472-6882-13-37
Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA, Kandaswami (2006) The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-κβ system. Clin Vaccine Immunol 13(3):319–328
Ngo Bum E, Taiwe GS, Moto FC, Ngoupaye GT, Nkantchoua GC, Pelanken MM, Rakotonirina SV, Rakotonirina A (2009) Anticonvulsant, anxiolytic, and sedative properties of the roots of Nauclea latifolia Smith in mice. Epilepsy Behav 15(4):434–440. https://doi.org/10.1016/j.yebeh.2009.05.014
Njan AA, Adenuga FO, Ajayi AM, Sotunde O, Ologe MO, Olaoye SO, Erdogan ON, Iwalewa OE (2020) Neuroprotective and memory-enhancing effects of methanolic leaf extract of Peristrophe bicalyculata in rat model of type 2 diabetes mellitus. Heliyon 6(5):e04011
Nonmarmbaye R, Kouemou N, Neteydji S, Njapdounke JSK, Bum EN (2021) Mnemonic and neuroprotective properties of Sclerocarya birrea root bark decoction on mouse model of monosodium glutamate-induced neurotoxicity involve by its antioxidant activities. GSC Biol Pharm Sci 17(1):193–203
Obafemi TO, Olaleye MT, Akinmoladun AC (2019) Antidiabetic property of miracle fruit plant (Synsepalum dulcificum Shumach. & Thonn. Daniell) leaf extracts in fructose-fed streptozotocin-injected rats via anti-inflammatory activity and inhibition of carbohydrate metabolizing enzymes. J Ethnopharmacol 244:112124. https://doi.org/10.1016/j.jep.2019.112124
Papanas N, Ziegler D (2015) Risk factors and comorbidities in diabetic neuropathy: an update 2015. Rev Diabetic Stud 12(1–2):48
Parinandi NL, Thompson EW, Schmid HH (1990) Diabetic heart and kidney exhibit increased resistance to lipid peroxidation. Biochim Biophys Acta -Lipids Lipid Metab 1047(1):63–69
Punthakee Z, Goldenberg R, Katz P (2018) Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes 42(Suppl 1):S10-s15. https://doi.org/10.1016/j.jcjd.2017.10.003
Redish AD, Touretzky DS (1997) Cognitive maps beyond the hippocampus. Hippocampus 7(1):15–35. https://doi.org/10.1002/(sici)1098-1063(1997)7:1%3c15::Aid-hipo3%3e3.0.Co;2-6
Ryan RM, Deci EL (2006) Self-regulation and the problem of human autonomy: does psychology need choice, self-determination, and will? J Pers 74(6):1557–1585. https://doi.org/10.1111/j.1467-6494.2006.00420.x
Sadeghi A, Beigy M, Alizadeh S, Mazloom H, Vakili S, Ahmadi S, Meshkani R (2017) Synergistic effects of ad-libitum low-dose fructose drinking and low-dose streptozotocin treatment in Wistar rats: a mild model of type 2 diabetes. Acta Med Iran 55(5):304–310
Schmatz R, Mazzanti CM, Spanevello R, Stefanello N, Gutierres J, Corrêa M, da Rosa MM, Rubin MA, Schetinger MRC, Morsch VM (2009) Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur J Pharmacol 610(1–3):42–48
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47(2):389–394. https://doi.org/10.1016/0003-2697(72)90132-7
Sommerfield AJ, Deary IJ, McAulay V, Frier BM (2003) Short-term, delayed, and working memory are impaired during hypoglycemia in individuals with type 1 diabetes. Diabetes Care 26(2):390–396. https://doi.org/10.2337/diacare.26.2.390
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JC, Mbanya JC (2022) IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119
Tamaddonfard E, Farshid AA, Asri-Rezaee S, Javadi S, Khosravi V, Rahman B, Mirfakhraee Z (2013) Crocin improved learning and memory impairments in streptozotocin-induced diabetic rats. Iran J Basic Med Sci 16(1):91
Tang L-J, Li C, Hu S-Q, Wu Y-P, Zong Y-Y, Sun C-C, Zhang F, Zhang G-Y (2012) S-nitrosylation of c-Src via NMDAR-nNOS module promotes c-Src activation and NR2A phosphorylation in cerebral ischemia/reperfusion. J Mol Cell Biochem 365(1):363–377
Tiwari V, Kuhad A, Bishnoi M, Chopra K (2009) Chronic treatment with tocotrienol, an isoform of vitamin E, prevents intracerebroventricular streptozotocin-induced cognitive impairment and oxidative–nitrosative stress in rats. Pharmacol Biochem Behav 93(2):183–189
Tsikas D (2007) Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J Chromatogr B Analyt Technol Biomed Life Sci 851(1–2):51–70. https://doi.org/10.1016/j.jchromb.2006.07.054
Umegaki H (2014) Type 2 diabetes as a risk factor for cognitive impairment: current insights. Clin Interv Aging 9:1011–1019. https://doi.org/10.2147/cia.S48926
Van Handel E (1965) Estimation of glycogen in small amounts of tissue. Anal Biochem 11(2):256–265. https://doi.org/10.1016/0003-2697(65)90013-8
Welsh B, Wecker L (1991) Effects of streptozotocin-induced diabetes on acetylcholine metabolism in rat brain. Neurochem Res 16(4):453–460
Wilbur KM, Bernheim F, Shapiro OW (1949) The thiobarbituric acid reagent as a test for the oxidation of unsaturated fatty acids by various agents. Arch Biochem 24(2):305–313
Wilson RD, Islam MS (2012) Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol Rep 64(1):129–139
Xu Q-G, Li X-Q, Kotecha S, Cheng C, Sun H, Zochodne D (2004) Insulin as an in vivo growth factor. Exp Neurol 188(1):43–51
Zhao W-Q, Alkon DL (2001) Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol 177(1–2):125–134
Zhong Y, Zhu Y, He T, Li W, Yan H, Miao Y (2016) Rolipram-induced improvement of cognitive function correlates with changes in hippocampal CREB phosphorylation, BDNF and Arc protein levels. Neurosci Lett 610:171–176
Zilliox LA, Chadrasekaran K, Kwan JY, Russell JW (2016) Diabetes and cognitive impairment. Curr Diab Rep 16(9):1–11
Acknowledgements
The authors would like to acknowledge “Pathologie Cytologie Développement” for providing histological reagents. The authors are very thankful to the University of Yaoundé 1 (Cameroon) for their support in providing apparatus for behavioral testing.
Author information
Authors and Affiliations
Contributions
Conceptualization: Théophile Dimo, Florence Ngueguim Tsofack; Methodology: Jean Philippe Djientcheu Tientcheu, Florence Ngueguim Tsofack, Kandeda kavaye Antoine; Formal analysis and investigation: Jean Philippe Djientcheu Tientcheu, Rodrigue Fifen, Michel Arnaud Mbock; Writing – original draft preparation: Jean Philippe Djientcheu Tientcheu, Florence Ngueguim Tsofack; Writing – review and editing: Racelyne Kamkumo Gounoue, Théophile Dimo; Supervision: Théophile Dimo. All authors read and approved its final version.
Corresponding author
Ethics declarations
Ethics declarations
All the experiments were conducted in accordance with the approval of the Cameroon National Ethical Committee (Ref No. FwIRb00001954, 04/09/2006), which adopted the guidelines of the National Research Council's Guide for the Care and Use of Laboratory Animals. All animal experiments also comply with the ARRIVE guidelines 2.0.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The aqueous extract of the mixture of Sclerocarya birrea, Nauclea latifolia, and Piper longum (SNP) demonstrated neuroprotective and memory-strengthening properties by protecting hippocampus neurons in diabetic rats.
• Antidiabetic properties of the SNP mixture were exhibited via normalization of blood glucose levels and pancreas protection.
• SNP activity was due to the main naturally-occurring substances identified.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tientcheu, J.P.D., Ngueguim, F.T., Gounoue, R.K. et al. The extract of Sclerocarya birrea, Nauclea latifolia, and Piper longum mixture ameliorates diabetes-associated cognitive dysfunction. Metab Brain Dis 38, 2773–2796 (2023). https://doi.org/10.1007/s11011-023-01291-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-023-01291-7