Abstract
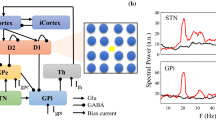
We examined electrical and optogenetic stimulations to explore their benefits and the effective range of their network effects. The error index (EI) and beta-activity of a network were considered by us as appropriate phenomena to compare stimulations. The basal ganglia (BG) network model considers areas of the brain affected by Parkinson’s disease (PD). It consists of the thalamus (TH), subthalamic nucleus (STN), globus pallidus interna (GPi,), and globus pallidus externa (GPe). To control the BG in PD, we stimulated the STN, GPe, and GPi with deep brain stimulation (DBS) and optogenetic stimulation, and to examine TH performance, we used the sensorimotor cortex (SMC). BG network performance was evaluated by measuring how TH responded to SMC input, and how STN, GPe, and Gpi were affected by DBS and optogenetic stimulation as evaluated using EI and beta-activity. By comparing the firing rates obtained from four applied stimulations at optimal conditions of the model, the effect of each stimulation was studied. The results indicated that applying monophasic DBS causes STN, GPi, and GPe cells to fire regularly, and biphasic DBS leads to increased oscillations between firings of STN cells; three-state optogenetic inhibition using halorhodopsin (NpHR) synchronizes the firing of GPe and GPi, and suppresses the neural activity of STN; and four-state optogenetic excitation using channelrhodopsin-2 (ChR2) incites the STN to excite and depolarize neural activity. All these events avoided pathological activation of PD and returned the performance to that of the healthy state. Finally, to evaluate our suggested method, we measured the beta-activity of monophasic and biphasic DBS and optogenetic inhibition and excitation by varying the \({\mathrm{electrical \ stimulation \ intensity }\ (A}_{elec})\) and optical stimulation intensity \(({A}_{light})\) and obtained optimal values for each state. According to our results, increasing \({A}_{elec}\) does not cause immediate decrease of beta-activity in monophasic and biphasic DBS. Increasing \({A}_{light}\) causes immediate decrease of beta-activity in NpHR, which then remains constant. In ChR2 there is no significant relation between beta-activity and \({A}_{light}.\)



















Similar content being viewed by others
References
Asadzade A and Miandoab SA 2021 Design and simulation of 3D perovskite solar cells based on titanium dioxide nanowires to achieve high-efficiency. Sol. Energy 228 550–561
Albaugh DL and Shih YY 2014 Neural circuit modulation during deep brain stimulation at the subthalamic nucleus for Parkinson’s disease: what have we learned from neuroimaging studies? Brain Connect 4 1–14
Appleby BS, Duggan PS, Regenberg A, et al. 2007 Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: A meta-analysis. Mov. Disord. 22 1722–1728
Alexander E, Evgenii G, Anastasia L, et al. 2019 Light stimulation parameters determine neuron dynamic characteristics. Appl. Sci. 9 3673
Bisaglia M, Filograna R, Beltramini M, et al. 2014 Are dopamine derivatives implicated in the pathogenesis of Parkinson’s disease? Ageing Res. Rev. 13 107–114
Brown P and Williams D 2005 Basal ganglia local field potential activity: character and functional significance in the human. Clin. Neurophysiol. 116 2510
Beylergil SB, Murray J, Noecker AM, et al. 2021 Effects of subthalamic deep brain stimulation on fixational eye movements in Parkinson’s disease. J. Comput. Neurosci. 49 345–356
Cardin JA, Carlén M, Meletis K, et al. 2010 Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protoc. 5 247–254
Ertugrul OF, Kaya Y, Tekin R, et al. 2019 Detection of Parkinson’s disease by shifted 1 Dimension local Binary Patterns from Gaif. Expert Syst. Appl. 56 156–163
Emmeric T, Roberrecht R and Thom ST 2018 Computational model of optogenetic neurostimulation. Master's dissertation, Ghent University, Belgium
Fan DG, Wang ZH and Wang QY 2016 Optimal control of directional deep brain stim- ulation in the parkinsonian neuronal network. Commun. Nonlinear Sci. Numer. Simul. 36 219–237
Guiyeom K and Madeleine ML 2009 A model of pathological oscillations in the basal ganglia and deep brain stimulation in Parkinson’s disease. 31st Annual International Conference of the IEEE EMBS Minneapolis, Minnesota, USA
Girasole AE, Lum MY, Nathaniel D, et al. 2018 A subpopulation of striatal neurons mediates levodopa-induced dyskinesia. Neuron 97 787–795
Glock C, Nagpal J and Gottschalk A 2015 Microbial rhodopsin optogenetic tools: Application for analyses of synaptic transmission and of neuronal network activity in behavior. Methods Mol. Biol. 2468 89–115
Gunaydin L, Yizhar O, Berndt A, et al. 2010 Ultrafast optogenetic control. Nat. Neurosci. 13 387–392
Gradinaru V, Thompson KR, Deisseroth K, et al. 2008 eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 36 129–139
Hurtado JM, Rubchinsky LL, Sigvardt KA, et al. 2004 Temporal evolution of oscillations and synchrony in GPi/muscle pairs in Parkinson’s disease. J. Neurophysiol. 93 1569–1584
Herrington TM, Cheng JJ and Eskandar EN 2016 Mechanisms of deep brain stimulation. J. Neurophysiol. 115 19–38
Honghui Z, Ying Y, Zichen D, et al. 2020 Activity pattern analysis of the subthalamopallidal network under ChannelRhodopsin-2 and Halorhodopsin photocurrent control. Chaos Solitons Fractals 138 109963
Hegemann P, Ehlenbeck S and Gradmann D 2005 Multiple photocycles of channelrhodopsin. Biophys. J. 89 3911–3918; Erratum in Biophys. J. 90 710
Josh D, Khajuria A and Joshi P 2017 An automatic non-invasive method for Parkinson’s disease classification. Comput. Methods Programs Biomed. 145 135–145
Kühn AA, Kempf F, Brücke C, et al. 2008 High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta-activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J. Neurosci. 28 6165–6173
Kühn AA, Tsui A, Aziz T, et al. 2009 Pathological synchronization in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp. Neurol. 215 380–387
Martinez-Martin P, Blazquez CR, Kurtis MM, et al. 2011 The impact of nonmotor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov. Disord. 26 399–406
Miandoab SA and Talebzadeh R 2022 Ultra-sensitive and selective 2D hybrid highly doped semiconductor-graphene biosensor based on SPR and SEIRA effects in the wide range of infrared spectral. Opt. Mater. 129 112572
Neumann W-J, Staub F, Horn A, et al. 2016 Deep brain recordings using an implanted pulse generator in Parkinson’s disease. Neuromodulation 19 20–24
Nir G, Konstantin N, Christofer T, et al. 2011 Modeling study of the light stimulation of a neuron cell with channelrhodopsin-2 mutants. IEEE Trans. Biomed. Eng. 58 1742–1751
Nagel G, Szellas T, Huhn W, et al. 2003a Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 100 13940–13945
Nagel G, Ollig D, Fuhrmann M, et al. 2002 Channelrhodopsin-1: A light-gated proton channel in green algae. Science 296 2395–2398
Nagel G, Szellas T, Huhn W, et al. 2003b Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 100 13940–13945
Prashanth R and Dutta Roy S 2018 Early detection of Parkinson’s disease through patent questionnaire and predictive modeling. Int. J. Med. Inform. 119 75–87
Park C, Worth RM and Rubchinsky LL 2011 Neural dynamics in parkinsonian brain: the boundary between synchronized and nonsynchronized dynamics. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 83 042901
Parter KL, Kim Y, Alberico SL, et al. 2016 Optogenetic approaches to evaluate striatal function in animal models of Parkinson disease. Dialogues Clin. Neurosci. 18 99–107
Pirini M, Rocchi L, Sensi M, et al. 2008 A computational modelling approach to investigate different targets in deep brain stimulation for Parkinson’s disease. J. Comput. Neurosci. 26 91–107
Ratnadurai-Giridharan S, Cheung CC and Rubchinsky LL 2017 Effects of electrical and optogenetic deep brain stimulation on synchronized oscillatory activity in Parkinsonian basal ganglia. IEEE Trans. Neural. Syst. Rehabil. Eng. 25 2188–2195
Rubin JE and Terman D 2004 High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J. Comput. Neurosci. 16 211–235
Schoenlein RW, Peteanu LA, Mathies RA, et al. 1991 The first step in vision: femtosecond isomerization of rhodopsin. Science 254 412–415
Schoeters R, Tarnaud T, Joseph W, et al. 2018 Comparison between direct electrical and optogenetic subthalamic nucleus stimulation. 2018 EMF-Med 1st World Conference on Biomedical Applications of Electromagnetic Fields (EMF-Med), Split, Croatia
So RQ, Kent AR and Grill WM 2012 Relative contributions of local cell and passing fiber activation and silencing to changes in thalamic fidelity during deep brain stimulation and lesioning: a computational modeling study. J. Comput. Neurosci. 32 499–519
Stefanescu RA, Shivakeshavan RG, Khargonekar PP, et al. 2013 Computational modeling of channelrhodopsin-2 photocurrent characteristics in relation to neural signaling. Bull. Math. Biol. 75 2208–2240
Stuart S, Alcock L, Galan B, et al. 2014 The measurement of visual sampling during real-world activity in Parkinson’s disease and healthy controls: a structured literature review. J. Neurosci. Methods 222 175–188
Sanders TH and Jaeger D 2016 Optogenetic stimulation of corticosubthalamic projections is sufficient to ameliorate bradykinesia in 6-ohda lesioned mice. Neurobiol. Dis. 95 225–237
Son YK, Park H, Firth AL, et al. 2013 Side-effects of protein kinase inhibitors on ion channels. J. Biosci. 38 937–949
Terman D, Rubin JE, Yew AC, et al. 2002 Activity patterns in a model for the subthalamopallidal network of the basal ganglia. J. Neurosci. 22 2963–2976
Tønnesen J 2015 Optogenetic cell control in experimental models of neurological disorders. Behav. Brain Res. 255 35–43
Umemura A, Jaggi JL, Hurtig HI, et al. 2003 Deep brain stimulation for movement disorders: morbidity and mortality in 109 patients. J. Neurosurg. 98 779–784
Wang Q, Schoenlein RW, Peteanu LA, et al. 1994 Vibrationally coherent photochemistry in the femtosecond primary event of vision. Science 266 422–424
Williams JC, Xu J, Lu Z, et al. 2013 Computational optogenetic: empirically-derived voltage- and light-sensitive channelrhodopsin-2 model. PLoS Comput. Biol. 9 17–19
Wingeier B, Tcheng T, Koop MM, et al. 2006 Intra-operative STN DBS attenuates the prominent beta rhythm in the STN in Parkinson’s disease. Exp. Neurol. 197 244–251
Yuvaraj R, Murugappan M, Ibrahim NM, et al. 2014a Optimal set of EEG features for emotional state classification and trajectory visualization in Parkinson’s disease. Int. J. Psychophysiol. 94 482–495
Yuvaraj R, Murugappan M, Ibrahim NM, et al. 2014b Detection of emotions in Parkinson’s disease using higher order spectral features from brain’s electrical activity. Biomed. Signal Process. Control 14 108–116
Yu C, Cassar IR, Sambangi J, et al. 2020 Frequency-specific optogenetic deep brain stimulation of subthalamic nucleus improves Parkinsonian motor behaviors. J. Neurosci. 40 4323–4334
Yu Y, Han F, Wang Q, et al. 2021 Model-based optogenetic stimulation to regulate beta oscillations in Parkinsonian neural networks. Cogn. Neurodyn. https://doi.org/10.1007/s11571-021-09729-3
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Corresponding editor: Aurnab Ghose
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghasemzadeh, N., Rahatabad, F.N., Haghipour, S. et al. Controlling pathological activity of Parkinson basal ganglia based on excitation and inhibition optogenetic models and monophasic and biphasic electrical stimulations. J Biosci 48, 40 (2023). https://doi.org/10.1007/s12038-023-00359-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-023-00359-x