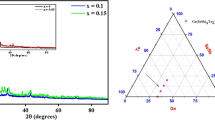
Phase equilibria and structural transformations in the La2O3–Y2O3–Gd2O3 system at 1600°C were studied by X-ray diffraction, electron microscopy, and petrography in the entire composition range. Fields of solid solutions based on hexagonal (A) modification of La2O3, cubic (C) modification of Y2O3, and monoclinic (B) modification of La2O3 (Gd2O3) were identified in the system. The starting materials were La2O3, Gd2O3, and Y2O3 (99.99%) powders. Samples were prepared with concentration steps of 1–5 mol.%. Weighed portions of the oxides were dissolved in HNO3 (1 : 1) solutions. This was followed by evaporation of the solutions and decomposition of the nitrates at 800°C for 2 h. The samples were heat-treated in three stages: 1100°C (168 h), 1500°C (70 h), and 1600°C (10 h) in air in furnaces with FeCrAl (H23U5T) and molybdenum disilicide (MoSi2) heating elements. X-ray diffraction analysis was carried out using the powder method with a DRON-3 diffractometer at room temperature (Cu-Kα radiation). The scanning step was 0.05–0.1° at angles 2θ = 15–90°. The isothermal section of the La2O3–Y2O3–Gd2O3 phase diagrams at 1600°C was characterized by three single-phase (A-La2O3, B-La2O3 (Gd2O3), C-Y2O3) and two two-phase (A + B, B + C) regions. The solubility limits were determined, and composition dependences of the lattice parameters for the phases formed in the system were plotted. No ordered perovskite-type phase was found in the system at 1600°C. A continuous series of solid solutions based on the monoclinic modification of B-La2O3(Gd2O3) formed in the system and occupied the largest area of the isothermal section. Yttrium oxide stabilized the total mutual solubility of lanthanum and gadolinium oxides. With the addition of heavier ions, the lattice parameters of the B modification reduced and the lattice volume and, accordingly, density increased. The lattice of solid solutions based on the B modification of rare-earth metal oxides became more densely packed with a higher concentration of yttrium oxide.









Similar content being viewed by others
References
S.F. Wang, J. Zhang, D.W. Luo, F. Gu, D.Y. Tang, Z.L. Dong, G.E.B. Tan, W.X. Que, T.S. Zhang, S. Li, and L.B. Kong, “Transparent ceramics: Processing, materials and applications,” Prog. Solid State Chem., 41, No. 1–2, 20–54 (2013).
J. Sanghera, S. Bayya, G. Villalobos, W. Kim, J. Frantz, B. Shaw, B. Sadowski, R. Miklos, C. Baker, M. Hunt, I. Aggarwal, and F. Kung, “Transparent ceramics for high-energy laser systems,” Opt. Mater., 33, 511–518 (2011).
M. Boniecki, Z. Librant, A. Wajler, W. Wesołowski, and H. Weglarz, “Fracture toughness, strength and creep of transparent ceramics at high temperature,” Ceram. Int., 38, No. 6, 4517–4524 (2012).
G.A. Vydrik, T.V. Solovieva, and F.Ya. Kharitonov, Transparent Ceramics [in Russian], Energiya, Moscow (1980), p. 96.
N.S. Prasad, S. Trivedi, K. Susan, Ch.-Ch. Wang, J.-S. Kim, U. Hommerich, V. Shukla, and R. Sadangi, “Development of ceramic solid-state laser host materials,” in: Proc. SPIE (2009), Vol. 7193, doi: https://doi.org/10.1117/12.813785.
S. Chen and Y. Wu, “New opportunities for transparent ceramics,” Am. Ceram. Soc. Bull., 92, No. 2, 32–37 (2013).
Y. Chen, X. Lin, Y. Lin, and Z. Luo, “Spectroscopic properties of Yb3+ ions in La2(WO4)3 crystal,” Solid State Comm., 132, No. 8, 533–538 (2004).
X. Gong, F. Xiong, and Y. Lin, “Crystal growth and spectral properties of Pr3+: La2(WO4)3,” Mater. Res. Bull., 42, No. 3, 413–419 (2007).
N. Lakshminarasimhan and U.V. Varadaraju, “Luminescent host lattices, LaInO3 and LaGaO3–A reinvestigation of luminescence of d10 metal ions,” Mater. Res. Bull., 41, No. 4, 724–731 (2006).
Masahiro Yoshimura and Xiao-Zheng Rong, “Various solid solutions in the systems Y2O3–R2O3 (R—La, Nd, and Sm) at high temperature,” J. Mater. Sci. Lett., 16, 1961–1963 (1997).
E.R. Andrievskaya, Phase Equilibria in Systems of Hafnium, Zirconium, and Yttrium Oxides with Rare Earth Oxides: Monograph [in Russian], Naukova Dumka, Kyiv (2010), p. 470.
E.R. Andrievskaya, “Phase equilibria in the refractory oxide systems of zirconia, hafnia and yttria with rare-earth oxides,” J. Eur. Ceram. Soc., 28, No. 12, 2363–2388 (2008).
J. Coutures, A. Rouanet, R. Verges, and M. Foex, “High-temperature study of systems formed by lanthanum sesquioxide and lanthanide sesquioxides. I. Phase diagrams (1400°C < T < T liquid),” J. Solid State Chem., 17, No. 1–2, 172–182 (1976).
J. Coutures, F. Sibieude, and M. Foex, “High-temperature study of systems formed by lanthanum sesquioxides with lanthanide sesquioxides. II. Influence of quenching on the nature of the phases obtained at room temperature,” J. Solid State Chem., 17, Issue 4, 377–384 (1976).
L.M. Lopato, B.S. Nigmanov, A.V. Shevchenko, and Z.A. Zaitseva, “Interaction of lanthanum oxide with yttrium oxide,” Izv. Akad Nauk SSSR Neorg. Mater., 22, No. 5, 771–774 (1986).
V. Berndt, D. Maier, and C. Keller, “New A”'B”'O3 interlanthanide perovskite compounds,” J. Solid State Chem., 13, No. 1–2, 131–135 (1975).
M. Mizuno, A. Rouanet, T. Yamada, and T. Noguchi, “Phase diagram of the system La2O3–Y2O3 at high temperatures,” J. Ceram. Soc. Jpn., 84, No. 7, 342–347 (1976).
J. Coutures and M. Foex, “High-temperature study of the equilibrium diagram of the system formed by yttrium sesquioxide,” J. Solid State Chem., 11, No. 4, 294–300 (1974).
G.C. Wei, T. Emma, and W.H. Rhodes, “Analytical microscopy study of phases and fracture in Y2O3–La2O3 alloys,” J. Am. Ceram. Soc., 71, No. 10, 820–825 (1988).
W.H. Rhodes, “Controlled transient solid second phase sintering of yttria,” J. Am. Ceram. Soc., 64, No. 1, 13–17 (1981).
A.V. Shevchenko, B.S. Nigmanov, Z.A. Zaitseva, and L.M. Lopato, “Interaction of samarium and gadolinium oxides with yttrium oxide,” Izv. Akad. Nauk SSSR Neorg. Mater., 22, No. 5, 775–778 (1986).
M. Zinkevich, “Thermodynamics of rare earth sesquioxides,” Prog. Mater. Sci., 52, 597–647 (2007).
S.A. Toropov, Phase Diagrams of Refractory Systems [in Russian], Nauka, Leningrad (1987), p. 822.
Y. Zhang, Thermodynamic Properties of Rare Earth Sesquioxide, Supervisor: Prof In-ho Jung, McGill University, Montreal, QC, Canada (2016).
R. Horyń, E. Bukowska, and A. Sikora, “Phase relations in La2O3–Gd2O3–CuO system at 950°C,” J. Alloys Compd., 416, 209–213 (2006).
S.J. Schneider and R.S. Roth, “Phase equilibria in systems involving the rare-earth oxides. Part II. Solid state reactions in trivalent rare-earth oxide systems,” J. Res. Nat. Bur. Stand. A. Phys. Chem., 4, 317–332 (1960).
E.R. Andrievskaya, O.A. Kornienko, and O.I. Bykov, “Interaction of lanthanum and gadolinium oxides at 1100°C,” Sovr. Probl. Fiz. Materialoved., 26, 23–30 (2017).
M. Stopyra, I. Saenko, M. Ilatovskaia, and G. Savinykh, “Phase relations in the ZrO2–La2O3–Gd2O3 system: Experimental studies and phase modeling,” J. Am. Ceram. Soc., 102, No. 12, 7628–7644 (2019).
O.A. Korniienko, O.I. Bykov, A.V. Sameljuk, Yu.M. Bataiev, and S.V. Yushkevych, “Phase equilibria in the CeO2–La2O3–Gd2O3 system at 1250 and 1500°C,” Int. Res. J. Multidiscip. Technovation, 3, Issue 4, 17–31 (2021).
O.V. Chudinovych, E.R. Andrievskaya, Zh.D. Bagatyriova, O.I. Olifan and L.M. Spasionova, “Interaction of lanthanum, yttrium, and neodymium oxides at 1600°C,” Visn. Odes. Nats. Univ. Ser. Khim., 22, Issue 2 (62), 82–94 (2017).
O.V. Chudinovych, S.F. Korichev, and E.R. Andrievskaya, “Interaction of yttrium, lanthanum, and samarium oxides at 1600°C,” Powder Metall. Met. Ceram., 58, No. 9–10, 599–607 (2020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 62, Nos. 1–2 (549), pp. 106–119, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chudinovych, O.V., Bykov, O.I. & Samelyuk, A.V. Interaction of Lanthanum, Yttrium, and Gadolinium Oxides at 1600°C. Powder Metall Met Ceram 62, 86–97 (2023). https://doi.org/10.1007/s11106-023-00372-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-023-00372-7