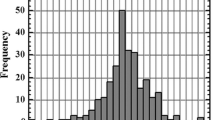
Mass spectra of molecular ions in the cathode sputtering of hydroxyapatite within argon glow discharges were studied experimentally and through mathematical simulation. The study was aimed at developing a highly sensitive technique for the determination of toxic and physiologically active elements in hydroxyapatite, used for medical purposes, by glow discharge mass spectrometry. Mass spectra were simulated employing the method developed previously for the calculation of molecular ion concentrations in glow discharge plasma and the computer program for its implementation. The effective equilibrium constants were refined for the formation–dissociation reactions of molecular ions during cathode sputtering of hydroxyapatite on a tantalum substrate. Comparison between the experimental and calculated mass spectra confirmed that the model was accurate. The study revealed molecular interferences in the mass range from 19F to 238U that were not adequately separated from the isotopes under study, thus reducing the analysis detection limit. The isotopes that were minimally affected by molecular interferences were chosen, and the resolution needed to achieve a detection limit of around 1 ppm for monoisotopic elements was calculated. To maintain a sufficiently high ionic current for nonconductive matrix isotopes (44Ca, 31P), a previously improved design of the analytical cell with high-purity tantalum as a substrate was employed. Most of the studied elements can be determined within ppm-ppb limits employing mass spectrometers with a high resolution (≥9000) at half the peak height.


Similar content being viewed by others
References
V.A. Dubok, “Bioceramics―yesterday, today, tomorrow,” Powder Metall. Met. Ceram., 39, No. 7–8, 381–394 (2000).
Y.G. Gogotsi and I.V. Uvarova, “Nanostructured materials and coatings for biomedical and sensor applications,” in: NATO Advanced Research Workshop on Nanostructured Materials and Coatings for Biomedical and Sensor Applications, Kyiv, Ukraine (2002).
S.V. Dorozhkin, “Calcium orthophosphates: occurrence, properties and major applications,” Bioceram. Dev., 4, Issue 2, 1000081 (2014), doi: https://doi.org/10.4172/2090-5025.1000081.
S.V. Dorozhkin, “Multiphasic calciumorthophosphate (CaPO4) bioceramics and their biomedical applications,” Ceram. Int., 42, 6529–6554 (2016).
F. Granados-Correa, J. Vilchis-Granados, M. Jiménez-Reyes, and L.A. Quiroz-Granados, “Adsorption behavior of La(III) and Eu(III) ions from aqueous solutions by hydroxyapatite: Kinetic, isotherm, and thermodynamic studies,” J. Chem., 2013, Article ID 751696 (2013), https://doi.org/10.1155/2013/751696.
Yan-zhong Zhao, Min Yang, Hai-bin Zhang, Jun Zhu, and Ke-chao Zhou, “Characterization of terbiumdoped nano-hydroxyapatite and surface modification,” J. Cent. South Univ., 23, No. 7, 1548–1555 (2016).
V.V. Garbuz, V.A. Dubok, L.F. Kravchenko, V.D. Kurochkin, N.V. Ul’yanchlch, and V.I. Kornilova, “Analysis of the chemical composition of a bioceramic based on hydroxyapatite and tricalcium phosphate,” Powder Metall. Met. Ceram., 37, No. 3–4, 193–195 (1998).
W. Schelles, S. De Gendt, and R. Van Grieken, “Optimization of secondary cathode thickness for direct current glow discharge mass spectrometric analysis of glass,” J. Anal. At. Spectrom., 11, 937–941 (1996).
W. Schelles, S. De Gendt, K. Maes, and R.V. Van Grieken, “The use of a secondary cathode to analyze solid non-conducting samples with direct current glow discharge mass spectrometry: potential and restrictions,” Fresenius J. Anal. Chem., 355, No. 7–8, 858–860 (1996).
V.D. Kurochkin, “Calculation of intensities of molecular interferences in GD-MS: Application to analysis of aluminum alloys,” Anal. Commun., 33, 381–384 (1996).
V.D. Kurochkin and L.P. Kravchenko, “Analysis of impurities in high-purity aluminum oxide by glow discharge mass spectrometry,” Powder Metall. Met. Ceram., 45, No. 9–10, 493–499 (2006).
V.D. Kurochkin, O.M. Romanenko, and M.O. Skulsky, “Determination of REE and some toxic elements in mine waters of Donetsk region by GD-MS,” Methods Objects Chem. Anal., 9, No. 2, 59–64 (2014).
S.L. Tong and W.W. Harrison, “Glow discharge mass spectrometric analysis of non-conducting materials,” Spectrochim. Acta, 48B, 1237–1245 (1993).
V.D. Kurochkin and O.M. Romanenko, “GDMS analysis of calcium carbonate naturally doped by REE, phosphates and other impurities,” Methods Objects Chem. Anal., 14, No. 2, 91–101 (2019).
V.D. Kurochkin, “Formation of ion dimeters from the cathode material in a cryo-cooled cell in glow discharge plasma,” Ukr. Khim. Zh., 65, No. 3, 57–63 (1999).
V.D. Kurochkin, “Formation of argon-containing molecular ions in cryo-cooled glow discharge plasma,” Ukr. Khim. Zh., 69, No. 9, 26–34 (2003).
Acknowledgments
The authors are grateful to I.B. Velykorydko for assistance in preparing the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 62, Nos. 1–2 (549), pp. 135–147, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kurochkin, V.D., Romanenko, O.M. Molecular Ion Spectra in Glow Discharge Mass Spectrometry of Toxic and Physiologically Active Elements in Hydroxyapatite. Powder Metall Met Ceram 62, 111–122 (2023). https://doi.org/10.1007/s11106-023-00374-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-023-00374-5