Abstract
Background
Carcinogenicity assessment of industrial chemicals is necessary because of the risk of workplaces exposure to these chemicals in humans.
Objectives
We aimed to evaluation nongenotoxicity and epigenetic carcinogenicity of the industrial chemicals anisol, 2-methoxyethanol, and 1,2-dichlorobenzene.
Results
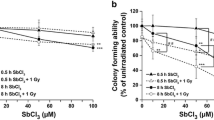
Treatment of Bhas 42 cells with these chemicals did not induce cell transformation, and there was no significant methylation difference in the methylation of CpG islands between the treatment and control groups.
Conclusion
Anisol, 2-methoxyethanol, and 1,2-dichlorobenzene do not exert nongenotoxic carcinogenicity or methylation-related epigenetic changes.




Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Baker SG, Cappuccio A, Potter JD (2010) Research on early-stage carcinogenesis: are we approaching paradigm instability? J Clin Oncol 28:3215–3218
Belpomme D et al (2007a) The multitude and diversity of environmental carcinogens. Environ Res 105:414–429
Belpomme D et al (2007b) The growing incidence of cancer: role of lifestyle and screening detection. Int J Oncol 30:1037–1049
Benigni R (2007) Social sexual inequality and sex difference in cancer incidence. Environ Res 104:128–134
Benigni R (2012a) Alternatives to the carcinogenicity bioassay for toxicity prediction: are we there yet? Exp Opin Drug Metab Toxicol 8:1–11
Benigni R (2012b) The New ISSMIC Database on in vivo micronucleus, and its role in assessing genotoxicity testing strategies. Mutagenesis 27:87–92
Benigni R, Bossa C (2011) Mechanisms of chemical carcinogenicity and mutagenicity: a review with implications for predictive toxicology. Chem Rev 111:2507–2536
Benigni R, Bossa C, Tcheremenskaia O, Giuliani A (2010) Alternatives to the carcinogenicity bioassay: in silico methods, and the in vitro and in vivo mutagenicity assays. Exp Opin Drug Metab Toxicol 6:1–11
Doll R, Peto R (1981) The Causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. Natl Cancer Inst 66:1192–1308
Du P et al (2010) Comparison of beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinform 11:1–9
EFSA Scientific Committee (2011) Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA J 9:2379
Elespuru RK et al (2009) Current and future application of genetic toxicity assays: the role and value of in vitro mammalian assays. Toxicol Sci 109:172–179
Goyal R, Reinhardt R, Jeltsch A (2006) Accuracy of DNA methylation pattern preservation by the Dnmt1 methyltransferase. Nucleic Acids Res 34:1182–1188
Greally JM, Jacobs MN (2013) In vitro and in vivo testing methods of epigenomic endpoints for evaluating endocrine disruptors. Altex 30:445–471
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Hattis D et al (2009) A preliminary operational classification system for nonmutagenic modes of action for carcinogenesis. Crit Rev Toxicol 39:97–138
Hayashi et al (2012) The validation management team report on the Bhas 42 CTA
Herceg Z et al (2013) Towards incorporating epigenetic mechanisms into carcinogen identification and evaluation. Carcinogenesis 34:1955–1967
Hernández LG, van Steeg H, Luijten M, van Benthem J (2009) Mechanisms of non-genotoxic carcinogens and importance of a weight of evidence approach. Mutat Res 682:94–109
Huff J (2008) More toxin tests needed. Science 319:725–726
ICH Guidance S2(R1) (ICH Geneva, Switzerland, 2008)
Infinium Mouse Methylation BeadChip Data Sheet, https://sapac.illumina.com/content/dam/ illumina/gcs/assembled-assets/marketing-literature/infinium-mouse-methylation-array-data-sheet-370-2020-002/infinium-mouse-methylation-array-data-sheet-370-2020-002.pdf
Kakunaga T (1973) A quantitative system for assay of malignant transformation by chemical carcinogens using a clone derived from Balb 3T3. Int J Cancer 12:463–473
Kirk T (1998) Kitchin in Carcinogenicity, 1st edn. CRC Press, Boca Raton
Kirkland D, Speit G (2008) Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens III. Appropriate follow-up testing in vivo. Mutat Res 654:114–132
Kirkland D, Aardema M, Henderson L, Müller L (2005) Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens I. Sensitivity, specificity and relative predictivity. Mutat Res 584:1–256
Lai DY, Woo YT (2012) Reducing carcinogenicity and mutagenicity through mechanism-based molecular design of chemicals. In Boethling RS and Voutchkova AM (Eds.), Green Processes. Volume 9: Designing Safer Chemicals. Wiley, Heidelberg
Liechtenstein et al (2000) Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343:78–85
Mascolo MG et al (2018) The transformics assay: first steps for the development of an integrated approach to investigate the malignant cell transformation in vitro. Carcinogenesis 39:955–967
Melnick RL, Kohn MC, Portier CJ (1996) Implications for risk assessment of suggested nongenotoxic mechanisms of chemical carcinogenesis. Environ Health Perspect 104:123–134
Meyer AL (1983) In vitro transformation assays for chemical carcinogens. Mutat Res 115:323–338
OECD Guidance Document No. 231 www.oecd.org/env/ehs/testing/ENV_JM_MONO(2016)1.pdf
Ohmori K, Kamei A, Watanabe Y, Abe K (2022) Gene expression over time during cell transformation due to non-genotoxic carcinogen treatment of Bhas 42 cells. Int J Mol Sci 23:3216
Pan Y, Liu G, Zhou F, Su B, Li Y (2018) DNA methylation profiles in cancer diagnosis and therapeutics. Clin Exp Med 18:1–14
Pfister SX, Ashworth A (2017) Marked for death: targeting epigenetic changes in cancer. Nat Rev Drug Discov 16:241–263
Portela A, Esteller M (2010) Epigenetic modifications and human disease. Nat Biotechnol 28:1057–1068
Sakai A et al (2010) A Bhas 42 cell transformation assay on 98 chemicals: the characteristics and performance for the prediction of chemical carcinogenicity. Mutat Re 702:100–122
Sakai A et al (2011) An international validation study of a Bhas 42 cell transformation assay for the prediction of chemical carcinogenicity. Mutat Res 725:57–77
Sasaki K, Mizusawa H, Ishidate M (1988) Isolation and characterization of ras-transfected BALB/3T3 clone showing morphological transformation by 12-O-tetradecanoylphorbol-13-acetate. Jpn J Cancer Res 79:921–930
Sasaki K, Umeda M, Sakai A, Yamazaki S, Tanaka N (2015) Transformation assay in Bhas 42 cells: a model using initiated cells to study mechanisms of carcinogenesis and predict carcinogenic potential of chemicals. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 33:1–35
Sokal RR, Oden NL, Rosenberg MS, Thomson BA (2000) Cancer incidences in Europe related to mortalities, and ethnohistoric, genetic, and geographic distances. Proc Natl Acad Sci 97:6067–6072
Steenland K, Burnett C, Lalich N, Ward E, Hurrell J (2003) Dying for work: the magnitude of US mortality from selected causes of death associated with occupation. Am J Ind Med 43:461–482
Tanaka N et al (2009) An interlaboratory collaborative study on a cell transformation assay using Bhas 42 cells. AATEX 14:831–848
Tomatis L et al (1997) Avoided and avoidable risks of cancer. Carcinogenesis 18:97–105
Waters MD, Jackson M, Lea I (2010) Characterizing and predicting carcinogenicity and mode of action using conventional and toxico genomics methods. Mutat Res 705:184–200
World Cancer Report 2014 (International Agency for Research on Cancer Lyon, France, 2014)
World Health Organization (WHO) (2023) Preventing cancer, www.who.int/activities/preventing-cancer
Zeiger E (1994) Strategies and philosophies of genotoxicity testing: what is the question? Mutat Res 304:309–314
Zeiger E (1998) Identification of rodent carcinogens and non-carcinogens using genetic toxicity tests: premises, promises, and performance. Regulat Toxicol Pharmacol 28:85–95
Zeiger E (2004) History and rationale of genetic toxicity testing: an impersonal, and sometimes personal, view. Environ Mol Mutagen 44:363–371
Acknowledgements
This study was conducted as an independent research project of the Occupational Safety and Health Research Institute in 2022-산업안전보건연구원-860, Dong Seok Seo, 2022-산업안전보건연구원-859, CheolHong Lim. SDS participated as Principal Investigator in the methylation assay. LCH participated as Principal Investigator in cell transformation assay. Also, we would like to thank Editage (www.editage.co.kr) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dong-Seok Seo declares that he has no conflict of interest. Cheol-Hong Lim declares that he has no conflict of interest.
Ethical approval
This study was not reviewed by the Institutional Animal Care and Use Committee because it did not involve animals and used only cells.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lim, C.H., Seo, D.S. Carcinogenicity assessment of industrial chemicals 1,2-dichlorobenzene, 2-methoxyethanol and anisol via Bhas 42 cell transformation assay. Mol. Cell. Toxicol. (2023). https://doi.org/10.1007/s13273-023-00402-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s13273-023-00402-w