Abstract
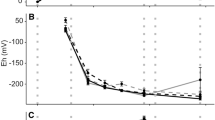
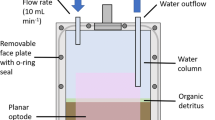
In flooded soils and sediments, bioturbating invertebrates rework sediment and convey oxygenated surface water through burrowing, creating a mosaic of adjacent anoxic and oxic patches while simultaneously translocating and transforming nutrients as they feed and excrete. We investigated the impacts of two functionally contrasting bioturbators (gallery-network burrower Lumbriculus variegatus and U-shaped burrower Ephemera simulans) on oxygen availability and nutrient fluxes in wetland sediments. To assess excretion contributions, we also incubated bioturbators in sand-water microcosms. Fine-scale oxygen measurements combined with flux rates of redox-sensitive and conservative ions reveal that both bioturbators introduced oxygen to sediments. U-shaped burrowers facilitated measurable oxygen introduction into sediments while gallery-network burrowers did not. However, gallery-network burrowers showed evidence of oxidizing reduced solutes in sediments, which suggests that oxygen is being introduced. At high densities, both bioturbators promoted sufficient iron oxidation to sequester phosphorus from surface water into sediments, effectively counteracting phosphorus release from excretion. Conversely, bioturbation caused nitrate release into surface water, likely driven by excretion of ammonia followed by nitrification. Gallery-network burrowers facilitated P retention in sediments but contributed N to surface water, while U-shaped burrowers showed similar, but less pronounced trends. Bioturbators have profound, but variable, effects on sediment-surface water nutrient exchange in wetlands. Sediment characteristics, bioturbator density, and bioturbation mode regulate both the amount of oxygen introduced to normally anoxic sediments and its reactions with sediment substrates, shaping the magnitude and direction of bioturbator-induced nutrient fluxes.




Similar content being viewed by others
References
Aminot A, Kirkwood DS, Kérouel R (1997) Determination of ammonia in seawater by the indophenol-blue method: evaluation of the ICES NUTS I/C 5 questionnaire. Mar Chem 56:59–75
Anderson DM, Glibert PM, Burkholder JM (2002) Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries 25:704–726
Baranov V, Lewandowski J, Krause S (2016) Bioturbation enhances the aerobic respiration of lake sediments in warming lakes. Biol Lett 12:1–4
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear Mixed-Effects Models using lme4. J Stat Softw 67:1–48
Batzer DP, Rader RB, Wissinger SA (1999) Invertebrates in Freshwater Wetlands of North America: Ecology and Management. John Wiley & Sons, Inc, New York
Behney AC, O’Shaughnessy R, Eichholz MW, Stafford JD (2014) Influence of item distribution pattern and abundance on efficiency of Benthic Core Sampling. Wetlands 34:1109–1121
Biles CL, Paterson DM, Ford RB, Solan M, Raffaelli DG (2002) Bioturbation, ecosystem functioning and community structure. Hydrol Earth Syst Sci 6:999–1005
Blouin MA, Hudson P, Chriscinske M (2004) Habitat Selection by two species of burrowing Mayfly Nymphs in the Les Cheneaux Islands Region of Northern Lake Huron. J Freshw Ecol 19:507–514
Boeker C, Lueders T, Mueller M, Pander J, Geist J (2016) Alteration of physico-chemical and microbial properties in freshwater substrates by burrowing invertebrates. Limnologica 59:131–139
Caliman A, Leal JJF, Esteves FA, Carneiro LS, Bozelli RL, Farjalla VF (2007) Functional bioturbator diversity enhances benthic–pelagic processes and properties in experimental microcosms. J North Am Benthological Soc 26:450–459
Canfield DE, Farquhar J (2009) Animal evolution, bioturbation, and the sulfate concentration of the oceans. Proc Natl Acad Sci 106:8123–8127
Cariou M, Francois CM, Voisin J, Pigneret M, Hervant F, Volatier L, Mermillod-Blondin F (2021) Effects of bioturbation by tubificid worms on biogeochemical processes, bacterial community structure and diversity in heterotrophic wetland sediments. Sci Total Environ 795:148842
Chaffin JD, Kane DD (2010) Burrowing mayfly (Ephemeroptera: Ephemeridae: Hexagenia spp.) bioturbation and bioirrigation: a source of internal phosphorus loading in Lake Erie. J Great Lakes Res 36:57–63
Chatarpaul L, Robinson JB, Kaushik NK (1980) Effects of Tubificid Worms on Denitrification and Nitrification in Stream Sediment. Can J Fish Aquat Sci 37:656–663
Chen M, Ding S, Liu L, Wang Y, Xing X, Wang D, Gong M, Zhang C (2016a) Fine-scale bioturbation effects of tubificid worm (Limnodrilus hoffmeisteri) on the lability of phosphorus in sediments. Environ Pollut 219:604–611
Chen M, Ding S, Liu L, Xu D, Gong M, Tang H, Zhang C (2016b) Kinetics of phosphorus release from sediments and its relationship with iron speciation influenced by the mussel (Corbicula fluminea) bioturbation. Sci Total Environ 542:833–840
Costello DM, Hammerschmidt CR, Burton GA (2015) Copper sediment toxicity and partitioning during oxidation in a Flow-Through Flume. Environ Sci Technol 49:6926–6933
Cottingham KL, Lennon JT, Brown BL (2005) Knowing when to draw the line: Designing more informative ecological experiments. Front Ecol Environ 3:145–152
Cozzoli F, Gjoni V, Del Pasqua M, Hu Z, Ysebaert T, Herman PMJ, Bouma TJ (2019) A process based model of cohesive sediment resuspension under bioturbators’ influence. Sci Total Environ 670:18–30
Cozzoli F, Shokri M, Gomes da Conceição T, Herman PMJ, Hu Z, Soissons LM, Van Dalen J, Ysebaert T, Bouma TJ (2021) Modelling spatial and temporal patterns in bioturbator effects on sediment resuspension: a biophysical metabolic approach. Sci Total Environ 792:148215
Davenport ES, Shull DH, Devol AH (2012) Roles of sorption and tube-dwelling benthos in the cycling of phosphorus in Bering Sea sediments. Deep Sea Res Part II 65–70:163–172
Devine JA, Vanni MJ (2002) Spatial and seasonal variation in nutrient excretion by benthic invertebrates in a eutrophic reservoir: nutrient excretion by benthic invertebrates. Freshw Biol 47:1107–1121
Edgar GJ, Barrett NS (2002) Benthic macrofauna in tasmanian estuaries: scales of distribution and relationships with environmental variables. J Exp Mar Biol Ecol 270:1–24
Edsall TA, Haas RC, Adams JV (2001) Annual Production of burrowing Mayfly Nymphs (Hexagenia spp.) in U.S. Waters of Lake St. Clair. J Great Lakes Res 27:449–456
Fanjul E, Bazterrica MC, Escapa M, Grela MA, Iribarne O (2011) Impact of crab bioturbation on benthic flux and nitrogen dynamics of Southwest Atlantic intertidal marshes and mudflats. Estuarine, Coastal and Shelf Science 92:629–638
Gagnon J-M, Beaudin L, Silverberg N, Mauviel A (2013) Mesocosm and in situ observations of the burrowing shrimp Calocaris templemani (Decapoda: Thalassinidea) and its bioturbation activities in soft sediments of the Laurentian Trough. Mar Biol 160:2687–2697
Gallepp GW (1979) Chironomid Influence on Phosphorus Release in Sediment-Water Microcosms. Ecology 60:547–556
Gautreau E, Volatier L, Nogaro G, Gouze E, Mermillod-Blondin F (2020) The influence of bioturbation and water column oxygenation on nutrient recycling in reservoir sediments. Hydrobiologia 847:1027–1040
Geurts JJM, Smolders AJP, Verhoeven JTA, Roelofs JGM, Lamers LPM (2008) Sediment Fe:PO ratio as a diagnostic and prognostic tool for the restoration of macrophyte biodiversity in fen waters. Freshw Biol 53:2101–2116
Gilbert F, Aller RC, Hulth S (2003) The influence of macrofaunal burrow spacing and diffusive scaling on sedimentary nitrification and denitrification: an experimental simulation and model approach. J Mar Res 61:101–125
Gilbertson WW, Solan M, Prosser JI (2012) Differential effects of microorganism-invertebrate interactions on benthic nitrogen cycling. FEMS Microbiol Ecol 82:11–22
Granéli W (1979) The influence of chironomus plumosus larvae on the exchange of dissolved substances between sediment and water. Hydrobiologia 66:149–159
Grasshoff P (1983) Determination of nutrients. Pages 125–187 methods of seawater analysis. Verlag Chemie. FRG
Heath RT (1992) Nutrient Dynamics in Great Lakes Coastal Wetlands: future directions. J Great Lakes Res 18:590–602
Heling CL, Stelzer RS, Drecktrah HG, Koenigs RP (2018) Spatial variation of benthic invertebrates at the whole-ecosystem scale in a large eutrophic lake. Freshw Sci 37:605–617
Herdendorf CE (2006) In: Klarer DM, Herdendorf RC (eds) The ecology of Old Woman Creek, Ohio: an estuarine and watershed profile, 2nd edn. Ohio Dept. of Natural Resources, Columbus, OH
Howe RL, Rees AP, Widdicombe S (2004) The impact of two species of bioturbating shrimp (Callianassa subterranea and Upogebia deltaura) on sediment denitrification. J Mar Biol Association United Kingd 84:629–632
Jensen HS, Kristensen P, Jeppesen E, Skytthe A (1992) Iron:phosphorus ratio in surface sediment as an indicator of phosphate release from aerobic sediments in shallow lakes. Hydrobiologia 235/236:731–743
Jones CG, Lawton JH, Shachak M (1994) Organisms as Ecosystem Engineers 69:373–386
Kamp-Nielsen L (1974) Mud-water exchange of phosphate and other ions in undisturbed sediment cores and factors affecting the exchange rates. Archiv für Hydrobiologie 73:218–237
Krieger KA (1992) The Ecology of Invertebrates in Great Lakes Coastal Wetlands: current knowledge and research needs. J Great Lakes Res 18:634–650
Krieger KA, Bur MT, Ciborowski JJH, Barton DR, Schloesser DW (2007) Distribution and abundance of burrowing Mayflies (Hexagenia spp.) in Lake Erie, 1997–2005. J Great Lakes Res 33:20–33
Kristensen E, Penha-Lopes G, Delefosse M, Valdemarsen T, Quintana C, Banta G (2012) What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Mar Ecol Prog Ser 446:285–302
Kudrolli A, Ramirez B (2019) Burrowing dynamics of aquatic worms in soft sediments. Proc Natl Acad Sci 116:25569–25574
Kuypers MMM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16:263–276
Laverock B, Gilbert JA, Tait K, Osborn AM, Widdicombe S (2011) Bioturbation: impact on the marine nitrogen cycle. Biochem Soc Trans 39:315–320
Lawrence GB, Mitchell MJ, Landers DH (1982) Effects of the burrowing mayfly, Hexagenia, on nitrogen and sulfur fractions in lake sediment microcosms. Hydrobiologia 87:273–283
Lenth RV (2021) emmeans: Estimated Marginal Means, aka Least-Squares Means
Li Y, Yu S, Strong J, Wang H (2012) Are the biogeochemical cycles of carbon, nitrogen, sulfur, and phosphorus driven by the FeIII–FeII redox wheel in dynamic redox environments? J Soils Sediments 12:683–693
Matisoff G, Fisher JB, Matis S (1985) Effects of benthic macroinvertebrates on the exchange of solutes between sediments and freshwater. Hydrobiologia 122:19–33
Mermillod-Blondin F, Rosenberg R (2006) Ecosystem engineering: the impact of bioturbation on biogeochemical processes in marine and freshwater benthic habitats. Aquat Sci 68:434–442
Mermillod-Blondin F, Rosenberg R, François-Carcaillet F, Norling K, Mauclaire L (2004) Influence of bioturbation by three benthic infaunal species on microbial communities and biogeochemical processes in marine sediment. Aquat Microb Ecol 36:271–284
Mermillod-Blondin F, Nogaro G, Datry T, Malard F, Gibert J (2005) Do tubificid worms influence the fate of organic matter and pollutants in stormwater sediments? Environ Pollut 134:57–69
Michaud E, Desrosiers G, Mermillod-Blondin F, Sundby B, Stora G (2006) The functional group approach to bioturbation: II. The effects of the Macoma balthica community on fluxes of nutrients and dissolved organic carbon across the sediment–water interface. J Exp Mar Biol Ecol 337:178–189
Mitsch WJ, Reeder BC (1991) Modelling nutrient retention of a freshwater coastal wetland: estimating the roles of primary productivity, sedimentation, resuspension and hydrology. Ecol Model 54:151–187
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural water. Anal Chim Acta 27:31–36
Nogaro G, Burgin AJ (2014) Influence of bioturbation on denitrification and dissimilatory nitrate reduction to ammonium (DNRA) in freshwater sediments. Biogeochemistry 120:279–294
Norkko J, Reed DC, TimMermann K, Norkko A, Gustafsson BG, Bonsdorff E, Slomp CP, Carstensen J, Conley DJ (2012) A welcome can of worms? Hypoxia mitigation by an invasive species. Glob Change Biol 18:422–434
Politi T, Barisevičiūte R, Bartoli M, Bonaglia S, Cardini U, Castaldelli G, Kančauskaitė A, Marzocchi U, Petkuviene J, Samuiloviene A, Vybernaite-Lubiene I, Zaiko A, Zilius M (2021) A bioturbator, a holobiont, and a vector: the multifaceted role of Chironomus plumosus in shaping N‐cycling. Freshw Biol 66:1036–1048
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Reddy KR, DeLaune RD (2008) Biogeochemistry of wetlands: Science and applications. CRC Press
Renz J, Forster S (2014) Effects of bioirrigation by the three sibling species of Marenzelleria spp. On solute fluxes and porewater nutrient profiles. Mar Ecol Prog Ser 505:145–159
Samuiloviene A, Bartoli M, Bonaglia S, Cardini U, Vybernaite-Lubiene I, Marzocchi U, Petkuviene J, Politi T, Zaiko A, Zilius M (2019) The Effect of Chironomid Larvae on Nitrogen Cycling and Microbial Communities in Soft sediments. Water 11:1–16
Søndergaard M, Jensen JP, Jeppesen E (2003) Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 506–509:135–145
Soster FM, Matisoff G, McCall PL, Robbins JA (2001) In situ effects of organisms on porewater geochemistry in great lakes sediments. Pages 279–295 Organism-Sediment Interactions.
Svensson JM, Enrich-Prast A, Leonardson L (2001) Nitrification and denitrification in a eutrophic lake sediment bioturbated by oligochaetes. Aquat Microb Ecol 23:177–186
Szymańska M, Burandt P, Bąkowska M, Sowiński P, Mrozińska N, Obolewski K (2020) Long-term effects of hydromorphological stream restoration on changes in microhabitats of Ephemera danica (Ephemeroptera) and its population. Ecol Ind 109:105810
Tátrai I (1988) Experiments on Nitrogen and Phosphorus Release by Chironomus ex gr.plumosus from the sediments of Lake Balaton, Hungary. Int Revue der Gesamten Hydrobiol und Hydrographie 73:627–640
Thorp JH, Covich AP (2010) Ecology and classification of North American freshwater invertebrates. Third edition. Academic Press
Tuazon H, Kaufman E, Goldman DI, Bhamla MS (2022) Oxygenation-controlled collective Dynamics in aquatic worm blobs. Integr Comp Biol 62:890–896
Vanni MJ (2002) Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Syst 33:341–370
Vaughn CC, Hakenkamp CC (2001) The functional role of burrowing bivalves in freshwater ecosystems: functional role of bivalves. Freshw Biol 46:1431–1446
Verhoeven J, Arheimer B, Yin C, Hefting M (2006) Regional and global concerns over wetlands and water quality. Trends Ecol Evol 21:96–103
Villa J, Bohrer G, Kinsman-Costello LE, Ju Y (2022) Nutrient and carbon concentrations in dated soil cores at US-OWC Ameriflux wetland site (OWC NERR). Functional-type modeling approach and data-driven parameterization of methane emissions in wetlands. ESS-DIVE repository, Dataset
Volkenborn N, Polerecky L, Hedtkamp SIC, van Beusekom JEE (2007) and D. de Beer. Bioturbation and bioirrigation extend the open exchange regions in permeable sediments. Limnology and Oceanography 52:1898–1909
Volpers M, Neumann D (2003) Tolerance of two tubificid species (Tubifex tubifex and Limnodrilus hoffmeisteri) to hypoxic and sulfidic conditions in novel, long-term experiments. Archiv für Hydrobiologie 5:13–38
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Sci Total Environ 380:48–65
Wang F, Tessier A, Hare L (2001) Oxygen measurements in the burrows of freshwater insects: Oxygen in insect burrows. Freshw Biol 46:317–327
Webb A, Eyre B (2004) Effect of natural populations of burrowing thalassinidean shrimp on sediment irrigation, benthic metabolism, nutrient fluxes and denitrification. Mar Ecol Prog Ser 268:205–220
Welsh DT (2003) It’s a dirty job but someone has to do it: the role of marine benthic macrofauna in organic matter turnover and nutrient recycling to the water column. Chem Ecol 19:321–342
Wickham H (2009) ggplot2: Elegant graphics for data analysis
Wiesebron LE, Steiner N, Morys C, Ysebaert T, Bouma TJ (2021) Sediment bulk density effects on benthic macrofauna burrowing and bioturbation behavior. Front Mar Sci 8:1–16
Zedler JB, Kercher S (2005) WETLAND RESOURCES: Status, Trends, Ecosystem Services, and restorability. Annu Rev Environ Resour 30:39–74
Zilius M, Daunys D, Bartoli M, Marzocchi U, Bonaglia S, Cardini U, Castaldelli G (2022) Partitioning benthic nitrogen cycle processes among three common macrofauna holobionts. Biogeochemistry 157:193–213
Acknowledgements
This work was supported by Kent State University Research and Sponsored Programs, Old Woman Creek National Estuarine Research Reserve, Ohio Department of Natural Resources Office of Coastal Management and Ohio Sea Grant. We thank Old Woman Creek National Estuarine Research Reserve for facilitating our work, members of the Kinsman-Costello lab for their assistance, and N. Michael for artwork assistance.
Funding
This work was supported by Kent State University Research and Sponsored Programs, Old Woman Creek National Estuarine Research Reserve, Ohio Department of Natural Resources Office of Coastal Management and Ohio Sea Grant.
Author information
Authors and Affiliations
Contributions
TCM, DMC, and LEKC developed the study questions and experimental design. Field work was completed by TCM, and lab analyses were completed by TCM and ASF. TCM wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
13157_2023_1737_MOESM1_ESM.docx
Supplementary Material 1: Additional figures (Fig. S1-S3) and tables (Table S1-S4) depicting experimental design, mixed-effects model regressions by replicate, Repeated-Measures ANOVA statistical output, and flux rates
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Michael, T.C., Costello, D.M., Fitzgibbon, A.S. et al. Invertebrate Activities in Wetland Sediments Influence Oxygen and Nutrient Dynamics at the Sediment-water Interface. Wetlands 43, 96 (2023). https://doi.org/10.1007/s13157-023-01737-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-023-01737-9