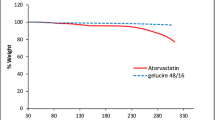
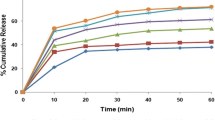
Abstract
Purpose
The goal of this study was to improve the solubility and bioavailability of poorly water-soluble atorvastatin calcium (ATC) by solid dispersion technique using natural polymer as a hydrophilic carrier. ATC is an anti-hyperlipidemic agent with low bioavailability due to its inability to dissolve in water. As a result, an effort has been undertaken to improve ATC’s oral bioavailability by making it more water-soluble using the solid dispersion approach.
Methods
Solid dispersions (SD) were prepared by using modified Samanea saman seed gum (MSSSG) as a natural hydrophilic carrier. Amongst various available methods, solvent evaporation method was selected to prepare solid dispersions. The chemical interaction between ATC and modified hydrophilic carrier was evaluated by FTIR spectroscopy.
Results
The result of the solubility study of ATC from solid dispersion found greater solubility of ATC compared to pure drug. The outcomes of an in vitro drug release study show higher cumulative drug release at 120 min, that is 96.95% of ATC from prepared solid dispersions in comparison to pure ATC, which was only 65.36%. The prepared solid dispersions were evaluated by DSC, XRD, and SEM studies. DSC and XRD study findings revealed that the crystalline drug was converted into an amorphous state. Pharmacokinetic study in rats showed approximately 1.68- and 2.3-fold increments in Cmax and AUC respectively in case of solid dispersion as compared to plain ATC.
Conclusion
In conclusion, MSSSG could be a promising carrier to improve the solubility, dissolution rate, and bioavailability of poorly water-soluble ATC.
Graphical Abstract







Similar content being viewed by others
Availability of Data and Materials
Not applicable.
Abbreviations
- ATC:
-
Atorvastatin calcium
- SD:
-
Solid dispersions
- MSSSG:
-
Modified Samanea saman seed gum
- NCE:
-
New chemical entities
- API:
-
Active pharmaceutical ingredient
- GI:
-
Gastrointestinal
- BCS:
-
Bio-pharmaceutical classification system
- FTIR:
-
Fourier transform infrared spectroscopy
- DSC:
-
Differential scanning calorimetry
- XRD:
-
X-ray diffraction
- SEM:
-
Scanning electron microscopy
- PM:
-
Physical mixture
- UV:
-
Ultra violet
- HPLC:
-
High-pressure liquid chromatography
- Cmax :
-
Maximum plasma concentration
References
Eesam S, Bhandaru JS, Akkinepally RR, Bobbala RK. Cocrystallization of gliclazide with improved physicochemical properties. Future J Pharm Sci. 2021;7(1). https://doi.org/10.1186/s43094-021-00261-z.
Thakuria R, Delori A, Jones W, Lipert MP, Roy L, Rodriguez-Hornedo N. Pharmaceutical cocrystals and poorly soluble drugs. Int J Pharm. 2013;453(1):101–25. https://doi.org/10.1016/j.ijpharm.2012.10.043.
Patole T. Co-Crystallization - A technique for solubility enhancement. Int J Pharm Sci Res. 5(9):3566–76. https://doi.org/10.13040/IJPSR.0975-8232.5(9).3566-76.
Al-Kazemi R, Al-Basarah Y, Nada A. Dissolution enhancement of atorvastatin calcium by cocrystallization. Adv Pharm Bull. 9(4):559–70. https://doi.org/10.15171/apb.2019.064.
Kumar S, Nanda A. Pharmaceutical cocrystals: An overview. Indian J Pharm Sci. 79(6). https://doi.org/10.4172/pharmaceutical-sciences.1000302.
Yuvaraja K, Khanam J. Enhancement of carvedilol solubility by solid dispersion technique using cyclodextrins, water soluble polymers and hydroxyl acid. J Pharm Biomed Anal. 2014;96:10–20. https://doi.org/10.1016/j.jpba.2014.03.019.
Jinno J, Kamada N, Miyake M, Yamada K, Mukai T, Odomi M, Toguchi H, Liversidge GG, Higaki K, Kimura T. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in Beagle Dogs. J Control Release. 2006;111(1–2):56–64. https://doi.org/10.1016/j.jconrel.2005.11.013.
Singh S, Bhagel R, Yadav L. A review on solid dispersion. Int J Pharm Life Sci. 2011;2:1078–95.
Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004;272(1–2):1–10. https://doi.org/10.1016/j.ijpharm.2003.11.025.
Li W, Qing S, Zhi W, Yao H, Fu C, Niu X. The pharmacokinetics and anti-inflammatory effects of chelerythrine solid dispersions in vivo. J Drug Deliv Sci Technol. 2017;40:51–8. https://doi.org/10.1016/j.jddst.2017.05.023.
Pawar JN, Shete RT, Gangurde AB, Moravkar KK, Javeer SD, Jaiswar DR, Amin PD. Development of amorphous dispersions of artemether with hydrophilic polymers via spray drying: Physicochemical and in Silico Studies. Asian J Pharm Sci. 2016;11(3):385–95. https://doi.org/10.1016/j.ajps.2015.08.012.
Pradhan R, Kim SY, Yong CS, Kim JO. Preparation and characterization of spray-dried valsartan-loaded Eudragit® e Po solid dispersion microparticles. Asian J Pharm Sci. 2016;11(6):744–50. https://doi.org/10.1016/j.ajps.2016.05.002.
Frijlink HW, Eissens AC, Hefting NR, Poelstra K, Lerk CF, Meijer DK. The effect of parenterally administered cyclodextrins on cholesterol levels in the rat. Pharm Res. 1991;08:9–16. https://doi.org/10.1023/A:1015861719134.
Palem CR, Patel S, Pokharkar VB. Solubility and stability enhancement of atorvastatin by cyclodextrin complexation. PDA J Pharm Sci Technol. 2009;63:217–25 (PMID: 20069794).
Serajuddin ATM. Salt formation to improve drug solubility. Adv Drug Deliv Rev. 2007;59(7):603–16. https://doi.org/10.1016/j.addr.2007.05.010.
Engel GL, Farid NA, Faul MM, Richardson LA, Winneroski LL. Salt form selection and characterization of ly333531 mesylate monohydrate. Int J Pharm. 2000;198(2):239–47. https://doi.org/10.1016/S0378-5173(00)00350-1.
Ahjel SW, Lupuleasa D. Enhancement of solubility and dissolution rate of different forms of atorvastatin calcium in direct compression tablet formulas. Farmacia. 2009;57:290–300.
Devarajan PV, Sonavane GS. Preparation and in vitro/in vivo evaluation of gliclazide loaded Eudragit nanoparticles as a sustained release carriers. Drug Dev Ind Pharm. 2007;33(2):101–11. https://doi.org/10.1080/03639040601096695.
Patel H, Pandey N, Patel B, Ranch K, Bodiwala K, Vyas B. Enhancement of in vivo hypoglycemic effect of gliclazide by developing self-microemulsifying pellet dosage form. Future J Pharm Sci. 2020;6(1). https://doi.org/10.1186/s43094-020-00034-0.
Agrawal A, Kumar A, Gide P. Toxicity study of a self-nanoemulsifying drug delivery system containing N-methyl pyrrolidone. Drug Res. 2015;65(08):446–48. https://doi.org/10.1055/s-0034-1389985.
Kumar N, Chaurasia S, Patel RR, Khan G, Kumar V, Mishra B. Atorvastatin calcium encapsulated eudragit nanoparticles with enhanced oral bioavailability, safety and efficacy profile. Pharm Dev Technol. 2015;22(2):156–67. https://doi.org/10.3109/10837450.2015.1108983.
Batisai E. Solubility enhancement of antidiabetic drugs using a co-crystallization approach. Chemistry Open. 2021;10(12):1260–8. https://doi.org/10.1002/open.202100246.
Arafa MF, El-Gizawy SA, Osman MA, El Maghraby GM. Co-crystallization for enhanced dissolution rate of nateglinide: In vitro and in vivo evaluation. J Drug Deliv Sci Technol. 2017;38:9–17. https://doi.org/10.1016/j.jddst.2017.01.005.
Wicaksono Y, Wisudyaningsih B, Siswoyo TA. Enhancement of solubility and dissolution rate of atorvastatin calcium by co-crystallization. Trop J Pharm Res. 2017;16(7):1497. https://doi.org/10.4314/tjpr.v16i7.6.
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: Importance and enhancement techniques. ISRN Pharmaceutics. 2012;1–10. https://doi.org/10.5402/2012/195727.
Patel M, Tekade A, Gattani S, Surana S. Solubility enhancement of lovastatin by modified locust bean gum using solid dispersion techniques. AAPS PharmSciTech. 2008;9(4):1262–69. https://doi.org/10.1208/s12249-008-9171-4.
Sekiguchi K, Obi N, Ueda Y. Studies on absorption of eutectic mixture. II. Absorption of fused conglomerates of chloramphenicol and urea in rabbits. Chem Pharm Bull. 1964;12(2):134–44. https://doi.org/10.1248/cpb.12.134.
Sekiguchi K, Obi N. Studies on absorption of eutectic mixture. i. A comparison of the behavior of eutectic mixture of sulfathiazole and that of ordinary sulfathiazole in man. Chem Pharm Bull. 1961;9(11):866–72. https://doi.org/10.1248/cpb.9.866.
Craig DQM. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm. 2002;231(2):131–44. https://doi.org/10.1016/S0378-5173(01)00891-2.
Ahmed S, Nagia El, Hanaa AF, Waleed B. Solubility enhancement of poorly water soluble drug by solid dispersion technique. Asian J Res Chem. 2012;5:483–91.
Cid AG, Simonazzi A, Palma SD, Bermudez JM. Solid dispersion technology as a strategy to improve the bioavailability of poorly soluble drugs. Ther Deliv. 2019;10(6):363–82. https://doi.org/10.4155/tde-2019-0007.
Allawadi D, Singh N, Singh S, Arora S. Cheminform abstract: Solid dispersions: A review on drug delivery system and solubility enhancement. ChemInform. 2014;45(18). https://doi.org/10.1002/chin.201418290.
Willart JF, Descamps M. Solid state amorphization of pharmaceuticals. Mol Pharm. 2008;5(6):905–20. https://doi.org/10.1021/mp800092t.
Kim JS, Kim MS, Park HJ, Jin SJ, Lee S, Hwang SJ. Physicochemical properties and oral bioavailability of amorphous atorvastatin hemi-calcium using spray-drying and SAS process. Int J Pharm. 2008;359(1–2):211–9. https://doi.org/10.1016/j.ijpharm.2008.04.006.
Desager JP, Horsmans Y. Clinical pharmacokinetics of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors. Clin Pharmacokinet. 1996;31(5):348–71. https://doi.org/10.2165/00003088-199631050-00003.
Bobe KR, Subrahmanya CR, Suresh S, Gaikwad DT, Patil MD, Khade TS, Gavitre BB, Kulkarni VS, Gaikwad UT. Formulation and evaluation of solid dispersion of atorvastatin with various carriers. Int J compr pharm. 2011;2:1–6.
Das SK. Solid dispersions : An approach to enhance the bioavailability of poorly water-soluble drugs. Int J Pharmacol Pharm Technol. 2013;37–46. https://doi.org/10.47893/ijppt.2013.1006.
Vemavarapu C, Mollan MJ, Lodaya M, Needham TE. Design and process aspects of laboratory scale SCF particle formation systems. Int J Pharm. 2005;292(1–2):1–16. https://doi.org/10.1016/j.ijpharm.2004.07.021.
York P, Kompella UB, Shekunov BY. Supercritical fluid technology for drug product development. New York: Informa Healthcare; 2008.
Rogers TL, Johnston KP, Williams RO. Solution-based particle formation of pharmaceutical powders by supercritical or compressed fluid CO2 and cryogenic spray-freezing technologies. Drug Dev Ind Pharm. 2001;27(10):1003–15. https://doi.org/10.1081/DDC-100108363.
Cilla DD, Whitfield LR, Gibson DM, Sedman AJ, Posvar EL. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of Atorvastatin, an inhibitor of HMG-COA reductase, in healthy subjects. Clin Pharmacol Ther. 1996;60(6):687–95. https://doi.org/10.1016/s0009-9236(96)90218-0.
Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84(3):413–28. https://doi.org/10.1016/S0163-7258(99)00045-5.
Dixit AK, Singh RP, Singh S. Solid dispersion-a strategy for improving the solubility of poorly soluble drugs. Int J Res Pharm Biomed Sci. 2012;3:960–6.
Baste NS, Basarkar GD. Samanea saman: a novel mucoadhesive gum. Res J Pharmacogn Phytochem. 2021;57–62. https://doi.org/10.52711/0975-4385.2021.00010.
Shingne NS, Nagpure SV, Deshmane SV, Biyani KR. Modified Hupu gum: a novel application in solid dispersion containing pioglitazone HCl. Am J PharmTech Res. 2013;3:463–72.
Jangra S, Ahuja M, Kumar A. Evaluation of mucoadhesive property of Gum Ghatti. J Pharm Investig. 2013;43(6):481–7. https://doi.org/10.1007/s40005-013-0093-0.
Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Dev Ind Pharm. 2004;30(9):985–93. https://doi.org/10.1081/DDC-200037245.
Murali Mohan Babu GV, Prasad ChDS, Ramana Murthy KV. Evaluation of modified Gum Karaya as carrier for the dissolution enhancement of poorly water-soluble drug nimodipine. Int J Pharm. 2002;234(1–2):1–17. https://doi.org/10.1016/S0378-5173(01)00925-5.
Padalkar AN, Shahi SR, Kale AG, Thube M, Padalkar VA. Formulation and characterization of novel solid dispersions of hydrochlorothiazide by solvent evaporation technique. Asian J Biomed Pharm Sci. 2012;2:49–56.
Rodde MS, Divase GT, Devkar TB, Tekade AR. Solubility and bioavailability enhancement of poorly aqueous soluble atorvastatin:in vitro, ex vivo, and in vivo studies. BioMed Research International. 2014:1–10. https://doi.org/10.1155/2014/463895.
Hasnain MS, Nayak AK. Solubility and dissolution enhancement of ibuprofen by solid dispersion technique using PEG 6000-PVP K 30 combination carrier. Chemistry. 2012;21:118–32.
Ratnaparkhi MP, Chaudhari PD. Solubility enhancement of poorly water soluble drug using natural carrier. International Journal of Life Science and Pharma Research. 2017;7:9–18.
Aggarwal S, Gupta GD, Chaudhary S. Solubility and dissolution enhancement of poorly aqueous soluble drug atorvastatin calcium using modified gum karaya as carrier: In vitro-In vivo evaluation. Int J Drug Deliv. 2012;4:341. https://doi.org/10.5138/ijdd.v4i3.751.
Jahangiri A, Barzegar-Jalali M, Garjani A, Javadzadeh Y, Hamishehkar H, Afroozian A, Adibkia K. Pharmacological and histological examination of atorvastatin-PVP K30 solid dispersions. Powder Technol. 2015;286:538–45. https://doi.org/10.1016/j.powtec.2015.08.047.
Maurya D, Belgamwar V, Tekade A. Microwave induced solubility enhancement of poorly water soluble atorvastatin calcium. J. Pharm Pharmacol. 2010;62(11):1599–1606. https://doi.org/10.1111/j.2042-7158.2010.01187.x.
Balasubramaniam J, Bindu K, Rao VU, Ray D, Haldar R, Brzeczko AW. Effect of superdisintegrants on dissolution of Cationic Drugs. Dissolution Technol. 2008;15(2):18–25. https://doi.org/10.14227/dt150208p18.
Charumanee S, Okonoki S, Sirithunyalug J. Improvement of the dissolution rate of piroxicam by surface solid dispersion. CMU J. 2004;3:77–84.
Acknowledgements
The authors are really thankful to Zydus Cadila Healthcare Private Limited, Ahmadabad, India, for providing a gift sample of atorvastatin calcium. The authors are also grateful to Marathwada Mitra Mandal’s College of Pharmacy, Pune, for providing facilities and continuous guidance throughout the research.
Author information
Authors and Affiliations
Contributions
ART and SUM conceptualized and designed the research. Data acquisition was carried out by SUM and GMK. Experimental work was performed by the SUM and GMK. The animal study was carried out by ART, SUM, and GMK. All authors have contributed to the data interpretation. SUM and MPR were involved in the analysis of data by using statistics. The final draft manuscript was prepared by ART and MPR. All authors were equally involved in the critical revision of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The animal study protocol was approved by the IAEC (IAEC Protocol Number MMCOP/IAEC/07/2021 Dated December 30, 2021).
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tekade, A.R., Mathapati, S.U., Ratnaparkhi, M.P. et al. Bioavailability Enhancement of Poorly Aqueous Soluble Atorvastatin Calcium by Solid Dispersion Technique Using a Modified Natural Polymer as a Hydrophilic Carrier. J Pharm Innov 18, 2182–2195 (2023). https://doi.org/10.1007/s12247-023-09783-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09783-w