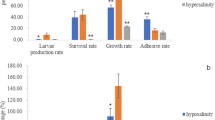
Abstract
Ocean acidification (OA) is a major stressor threatening marine calcifiers, including the eastern oyster (Crassostrea virginica). In this paper, we provide insight into the molecular mechanisms associated with resilience to OA, with the dual intentions of probing both acclimation and adaptation potential in this species. C. virginica were spawned, and larvae were reared in control or acidified conditions immediately after fertilization. RNA samples were collected from larvae and juveniles, and DNA samples were collected from juveniles after undergoing OA-induced mortality and used to contrast gene expression (RNAseq) and SNP (ddRADseq) profiles from animals reared under both conditions. Results showed convergence of evidence from both approaches, particularly in genes involved in biomineralization that displayed significant changes in variant frequencies and gene expression levels among juveniles that survived acidification as compared to controls. Downregulated genes were related to immune processes, supporting previous studies demonstrating a reduction in immunity from exposure to OA. Acclimation to OA via regulation of gene expression might confer short-term resilience to immediate threats; however, the costs may not be sustainable, underscoring the importance of selection of resilient genotypes. Here, we identified SNPs associated with survival under OA conditions, suggesting that this commercially and ecologically important species might have the genetic variation needed for adaptation to future acidification. The identification of genetic features associated with OA resilience is a highly-needed step for the development of marker-assisted selection of oyster stocks for aquaculture and restoration activities.





Similar content being viewed by others
Data Accessibility
Sequence reads are deposited in the SRA NCBI database and can be found under the BioProject ID PRJNA765289.
References
Aguilera F, McDougall C, Degnan BM (2017) Co-option and de novo gene evolution underlie molluscan shell diversity. Mol Biol Evol 34(4):779–792
Alexa A, Rahnenfuhrer J (2020) topGO: Enrichment analysis for gene ontology. R package version 2.40.0
Arivalagan J, Yarra T, Marie B, Sleight VA, Duvernois-Berthet E, Clark MS, Marie A, Berland S (2017) Insights from the shell proteome: biomineralization to adaptation. Mol Biol Evol 34:66–77
Barbosa M (2020) Physiological and molecular traits associated with resilience to ocean acidification in Crassostrea virginica. State University of New York at Stony Brook (Doctoral dissertation)
Barbosa M, Schwaner C, Pales Espinosa E, Allam B (2022) A transcriptomic analysis of phenotypic plasticity in Crassostrea virginica larvae under experimental acidification. Genes 13:1529. https://doi.org/10.3390/genes13091529
Barret DH, Schluter D (2008) Adaptation from standing genetic variation. Trends Ecol Evol 23:38–44
Barros P, Sobral P, Range P, Chicharo L, Matias D (2013) Effects of sea-water acidification on fertilization and larval development of the oyster Crassostrea gigas. J Exp Mar Biol Ecol 440:200–206
Baumann H, Wallace RB, Tagliaferri T, Gobler CJ (2015) Large natural pH, CO2, and O2 fluctuations in a temperate tidal salt marsh on diel, seasonal, and interannual time scales. Estuaries Coasts 38:220–231
Bay RA, Palumbi SR (2014) Multilocus adaptation associated with heat resistance in reef-building corals. Curr Biol 24:2952–2956
Beniash E, Ivanina A, Lieb NS, Kurochkin I, Sokolova IM (2010) Elevated level of carbon dioxide affects metabolism and shell formation in oysters Crassostrea virginica. Mar Ecol Prog Ser 419:95–108
Bibby R, Widdicombe S, Parry H, Spicer J, Pipe R (2008) Effects of ocean acidification on the immune response of the blue mussel Mytilus edulis. Aquat Biol 2:67–74
Bitter MC, Kapsenberg L, Gattuso JP, Pfister CA (2019) Standing genetic variation fuels rapid adaptation to ocean acidification. Nat Commun 10:5821. https://doi.org/10.1038/s41467-019-13767-1
Bitter MC, Kapsenberg L, Silliman K, Gattuso JP, Pfister CA (2021) Magnitude and predictability of pH fluctuations shape plastic responses to ocean acidification. Am Nat 197:486–501
Blank S, Arnoldi M, Khoshnavaz S, Treccani L, Kuntz M, Mann K, Grathwohl G, Fritz M (2003) The nacre protein perlucin nucleates growth of calcium carbonate crystals. J Microsc 212:280–291
Boutet I, Monteiro HJA, Baudry L, Takeuchi T, Bonnivard E, Billoud B, Farhat S, Gonzales-Araya R, Salaun B, Andersen AC, Toullec J, Lallier FH, Flot JF, Guiglielmoni N, Guo X, Li C, Allam B, Pales-Espinosa E, Hemmer-Hansen J, Moreau P, Marbouty M, Koszul R, Tanugy A (2022) Chromosomal assembly of the flat oyster (Ostrea edulis L.) genome as a new genetic resource for aquaculture. Evol Appl. https://doi.org/10.1111/eva.13462
Burgos-Aceves MA, Faggio C (2017) An approach to the study of the immunity functions of bivalve hemocytes: physiology and molecular aspects. Fish Shellfish Immunol 67:513–517
Calosi P, Rastrick SPS, Lombardi C, de Guzman HJ, Davidson L, Jahnke M, Giangrande A, Hardege JD, Schulze A, Spicer JI, Gambi MC (2013) Adaptation and acclimatization to ocean acidification in marine ectotherms: an in suite transplant experiment with polychaetes at a shallow CO2 vent system. Philos Trans R Soc B 368:2012044. https://doi.org/10.1098/rstb.2012.0444
Cai LS, Wang L, Song K, Lu KL, Zhang CX, Rahimnejad S (2020) Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture 516:734615
Cao R, Wang Q, Yang D, Liu Y, Ran W, Qu Y, Wu H, Cong M, Li F, Ji C, Zhao J (2018) CO2 -induced ocean acidification impairs the immune function of the Pacific oyster against Vibrio splendidus challenge: an integrated study from a cellular and proteomic perspective. Sci Total Environ 625:1574–1583. https://doi.org/10.1016/j.scitotenv.2018.01.056
Carreiro-Silva M, Cerqueira T, Godinho A, Caetano M, Santos RS, Bettencourt R (2014) Molecular mechanisms underlying the physiological responses of the cold-water coral Desmophyllum dianthus to ocean acidification. Coral Reefs 33:465–476
Castillo N, Saavedra LM, Vargas CA, Gallardo-Escarate C, Detree C (2017) Ocean acidification and pathogen exposure modulate the immune response of the edible mussel Mytilus chilensis. Fish Shellfish Immunol 70:149–155
Chegwidden WR, Carter ND, Edwards YH (2000) The carbonic anhydrases: new horizons. Birkhäuser, Basel
Choi Y, Sims GE, Murphy S, Miller JR, Chan AP (2012) Predicting the functional effect of amino acid substitutions and indels. PLoS ONE 7:e46688. https://doi.org/10.1371/journal.pone.0046688
Coban-Akdemir Z, White JJ, Song X, Jhangiani SN, Faith JM, Gambin T, Bayram Y, Chinn IK, Karaca E, Punetha J, Poli C, Boerwinkle E, Shaw CA, Orange JS, Gibbs RA, Lappalainen T, Lupski JR, Carvalho CMB (2018) Identifying genes whose mutant transcripts cause dominant disease traits by potential gain-of-function alleles. Am J Hum Genet 103:171–187
Cuttell L, Vaughan A, Silva E, Escaron CJ, Lavine M, Van Goethem E, Eid JP, Quirin M, Franc NC (2008) Undertaker, a Drosophila junctophilin, links draper-mediated phagocytosis and calcium homeostasis. Cell 135:524–534
DeWit P, Palumbi SR (2013) Transcriptome-wide polymorphisms of red abalone (Haliotis rufescens) reveal patterns of gene flow and local adaptation. Mol Ecol 22:2884–2897
De Wit P, Durland E, Ventura A, Langdon CJ (2018) Gene expression correlated with delay in shell formation in larval Pacific oysters (Crassostrea gigas) exposed to experimental ocean acidification provides insights into shell formation mechanisms. BMC Genomics 19:160. https://doi.org/10.1186/s12864-018-4519-y
Dineshram R, Quan Q, Sharma R, Chandramouli K, Yalamanchili HK, Chu I, Thiyagarjan V (2015) Comparative and quantitative proteomics reveal the adaptive strategies of oyster larvae to ocean acidification. Proteomics 15:4120–4134
Downey-Wall AM, Cameron LP, Ford BM, McNally EM, Venkataraman YR, Roberts SB, Ries JB, Lotterhos KE (2020) Ocean acidification induces subtle shifts in gene expression and DNA methylation in mantle tissue of the Eastern oyster (Crassostrea virginica). Front Mar Sci 7:566419. https://doi.org/10.3389/fmars.2020.566419
Dyachuk V (2018) Extracellular matrix components in Bivalvia: shell and ECM components in development and adult tissues. Fish Aqua J 9:2. https://doi.org/10.4172/2150-3508.1000248
Endo Y, Matsushita M, Fujita T (2007) Role of ficolin in innate immunity and its molecular basis. Immunobiology 212:371–379
Evans TG, Chang F, Menge BA, Hofmann GE (2013) Transcriptomic responses to ocean acidification in larval sea urchins from a naturally variable pH environment. Mol Ecol 22:1609–1625
Fabbri E, Valbonesi P, Franzellitti S (2008) HSP expression in bivalves ISJ 5:135–161
Farhat S, Tanguy A, Pales Espinosa E, Guo X, Boutet I, Smolowitz R, Murphy D, Rivara GJ, Allam B (2020) Identification of variants associated with hard clam, Mercenaria mercenaria, resistance to quahog parasite unknown disease. Genomics 112:4887–4896
Foll M, Gaggiotti OE (2008) A genome scan method to identify selected loci appropriate for both dominant and codominant markers: a Bayesian perspective. Genetics 180:977–993
Foulon V, Boudry P, Artiguad S, Guerard F, Hellio C (2019) In silico analysis of the Pacific oyster (Crassostrea gigas) transcriptome over developmental stages reveals candidate genes for larval settlement. Int J Mol Sci 20:197. https://doi.org/10.3390/ijms20010197
Frieder CA, Applebaum SL, Pan TCF, Hedgecock D, Manahan DT (2017) Metabolic cost of calcification in bivalve larvae under experimental ocean acidification. ICES J Mar Sci 74:941–954
Gallager SM (1988) Visual observation of particle manipulation during feeding in larvae of a bivalve mollusc. Bull Mar Sci 43:344–365
Gazeau F, Quiblier C, Jansen JM, Gattuso JP, Middleburg JJ, Heip CHR (2007) Impact of elevated CO2 on shellfish calcification. Geophys Res Lett 34:L07603. https://doi.org/10.1029/2006GL028554
Gazeau F, Gattuso JP, Dawber C, Pronker AE, Peene F, Peene J, Heip CHR, Middelburg JJ (2010) Effect of ocean acidification on the early life stages of the blue mussel Mytilus edulis. Biogeosciences 7:2051–2060
Gazeau F, Parker LM, Comeau S, Gattuso JP, O’Connor WA, Martin S, Pörtner HO, Ross PM (2013) Impacts of ocean acidification on marine shelled molluscs. Mar Biol 160:2207–2245
Glazier A, Herrera S, Weinning A, Kurman M, Gomez CE, Cordes E (2020) Regulation of ion transport and energy metabolism enables certain coral genotypes to maintain calcification under experimental ocean acidification. Mol Ecol 29:1657–1673
Gobler CJ, Talmage SC (2014) Physiological response and resilience of early life-stage Eastern oysters (Crassostrea virginica) to past, present and future ocean acidification. Conserv Physiol 2:cou004. https://doi.org/10.1093/conphys/cou004
Goncalves P, Anderson K, Thompson EL, Melwani A, Parker L, Ross PM, Raftos DA (2016) Rapid transcriptional acclimation following transgenerational exposure of oysters to ocean acidification. Mol Ecol 25:4836–4849
Goncalves P, Jones DB, Thompson EL, Parker LM, Ross PM, Raftos DA (2017) Transcriptomic profiling of adaptive responses to ocean acidification. Mol Ecol 26:5974–5988
Griffiths JS, Pan TF, Kelly MW (2019) Differential responses to ocean acidification between populations of Balanophyllia elegans corals from high and low upwelling environments. Mol Ecol 28:2715–2730
Grunwald NJ, Kamvar ZN, Everhart SE, Tabima JF, Kanus BJ (2017) Population genetics and genomics in R. Retrieved from https://grunwaldlab.github.io/Population_Genetics_in_R/. Accessed 30 Jul 2019
Helm MM, Bourne N, Lovatelli A (2004) Hatchery culture of bivalves: a practical manual. Food and Agriculture Organization of the United Nations, Rome
Hermisson J, Pennings PS (2005) Soft sweeps: molecular population genetics of adaptation from standing genetic variation. Genetics 169:2335–2352
Hernroth B, Baden S, Thorndyke M, Dupont S (2011) Immune suppression of the echinoderm Asterias rubens (L.) following long-term ocean acidification. Aquat 103:222–224
Hess JE, Campbell NR, Close DA, Docker MF, Narum SR (2013) Population genomics of Pacific lamprey: adaptive variation in a highly dispersive species. Mol Ecol 22:2898–2916
Hettinger A, Sanford E, Hill TM, Hosfelt JD, Russell AD, Gaylord B (2013) The influence of food supply on the response of Olympia oyster larvae to ocean acidification. Biogeosciences 10:6629–6638
Hüning AK, Melzner F, Thomsen J, Gutowska MA, Krämer L, Frickenhaus S, Rosenstiel P, Pörtner HO, Philipp EER, Lucassen M (2013) Impacts of seawater acidification on mantle gene expression patterns of the Baltic Sea blue mussel: implications for shell formation and energy metabolism. Mar Biol 160:1845–1861
Hunt S (1987) Amino acid compositions of periostracal proteins from molluscs living in the vicinity of deep sea hydrothermal vents: an unusual methionine-rich structural protein. Comp Biochem Phy b 88:1013–1021
Hunt R, Sauna ZE, Ambudkar SV, Gottesman MM, Kimchi-Sarfaty C (2009) Silent (synonymous) SNPs: should we care about them? Single nucleotide polymorphisms: methods and protocols. Humana Press, Totowa, NJ, pp 23–39
Johnson KM, Hofmann GE (2017) Transcriptomic response of the Antarctic pteropod Limacina helicina antarctica to ocean acidification. BMC Genomics 18(1):1–16
Jong MJ, Jong JF, Hoelzel AR, Janke A (2021) SambaR: an R package for fast, easy and reproducible population-genetic analyses of biallelic SNP datasets. Mol Ecol Resourc 21:1369–1379
Kapsenberg L, Miglioli A, Bitter MC, Tambutte E, Dumollard R, Gattuso JP (2018) Ocean pH fluctuations affect mussel larvae at key developmental transitions. Proc Royal Soc B 285:20182381. https://doi.org/10.1098/rspb.2018.2381
Kelly MW, Padilla-Gamiño JL, Hofmann GE (2013) Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Glob Change Biol 19:2536–2546
Kim D, Paggi JM, Park C, Bennett C, Salzberg SL (2019) Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37:907–915
Kocot KM, Aguilera F, McDougall C, Jackson DJ, Degnan BM (2016) Sea shell diversity and rapidly evolving secretomes: insights into the evolution of biomineralization. Front Zool 13:23
Kurihara H, Kato S, Ishimatsu A (2007) Effects of increased seawater pCO2 on early development of the oyster Crassostrea gigas. Aquat Biol 1:91–98
Larmuseau MHD, Vancampenhout K, Raeymaekers JAM, Van Houdt JKJ, Volckaert FAM (2010) Differential modes of selection on the rhodopsin gene in coastal Baltic and North Sea populations of the sand goby, Pomatoschistus minutus. Mol Ecol 19:2256–2268
Layton KK, Bradbury IR (2022) Harnessing the power of multi-omics data for predicting climate change response. J Anim Ecol 91(6):1064–1072
Lelong C, Mathieu M, Favrel P (2001) Structure and expression of mGDF, a new member of the transforming growth factor-B superfamily in the bivalve mollusc Crassostrea gigas. Eur J Biochem 267:3986–3993
Li S, Liu Y, Liu C, Huang J, Zheng G, Xie L, Zhang R (2015) Morphology and classification of hemocytes in Pinctada fucata and their responses to ocean acidification and warming. Fish Shellfish Immunol 45:194–202
Li S, Liu C, Huang J, Liu Y, Zhang S, Zheng G, Xie L, Zhang R (2016) Transcriptome and biomineralization responses of the pearl oyster Pinctada fucata to elevated CO2 and temperature. Sci Rep 6:18943. https://doi.org/10.1038/srep18943
Liu S, Shi W, Guo C, Zhao X, Han Y, Peng C, Chai X, Liu G (2016) Ocean acidification weakens the immune response of blood clam through hampering the NF-kappa β and toll-like receptor pathways. Fish Shellfish Immunol 54:322–327
Loosanoff VL (1966) Time and intensity of setting of the oyster, Crassostrea virginica, in Long Island Sound. Biol Bull 130:211–227
Lopez-Landavery EA, Carpizo-Ituarte EJ, Perez-Carrasco L, Diaz F, De le Cruz FL, Garcia-Esquivel Z, Hernandez-Ayon JM, Galindo-Sanchez CE (2021) Acidification stress effect on umbonate veliger larval development in panopea globosa. Mar Poll Bull 163:111945
Lowenstam HA, Weiner S (1989) On biomineralization. Oxford University Press, Oxford
Luu K, Bazin E, Blum MGB (2017) pcadapt: a R package to perform genome scans for selection based on principal component analysis. Mol Ecol Resour 1:67–77
Maas AE, Lawson G, Tarrant AM (2015) Transcriptome-wide analysis of the response of the thecosome pteropod clio pyramidata to short-term CO2 exposure. Comp Biochem Physiol Part d Genom Proteom 16:1–9
Manaka J, Kuraishi T, Shiratsuchi A, Nakai Y, Higashida H, Henson P, Nakanishi Y (2004) Draper-mediated and phosphatidylserine-independent phagocytosis of apoptotic cells by Drosophila hemoctyes/macrophages. J Biol Chem 279:48466–48476
Marie B, Joubert C, Tayalé A, Zanella-Cléon BC, Piquemal D, Cochennec-Laureau N, Marin F, Gueguen Y, Montagnani C (2012) Different secretory repertoires control the biomineralization processes of prism and nacre deposition of the pearl oyster shell. PNAS 109:20986–20991
Marie B, Arivalagan J, Matheron L, Bolbach G, Berland S, Marie A, Marin F (2017) Deep conservation of bivalve nacre proteins highlighted by shell matrix proteomics of the Unionoida Elliptio complanata and Villosa lienosa. J R Soc Interface 14:20160846. https://doi.org/10.1098/rsif.2016.0846
Marin F, Corstjens P, Gaulejac BD, de Vrind-De JE, Westbroek P (2000) Mucins and molluscan calcification molecular characterization of mucoperlin, a novel mucin-like protein from the nacreous shell layer of the fan mussel Pinna nobilis (Bivalvia, pteriomorphia). J Biol Chem 275:20667–20675
Martin S, Richier S, Pedrotti ML, Dupont S, Castejon C, Gerakis Y, Kerros M, Oberhansli F, Teyssie J, Jeffree R, Gattuso JP (2011) Early development and molecular plasticity in the Mediterranean sea urchin Paracentrotus lividus exposed to CO2-driven acidification. J Exp Biol 214(8):1357–1368
Metzger DC (2012) Characterizing the effects of ocean acidification in larval and juvenile Manilla clam, Ruditapes philippinarum, using a transcriptomic approach. University of Washington, Washington, DC, USA (Master’s Thesis)
Millero FJ (2010) Carbonate constants for estuarine waters. Mar Freshw Res 62:139–142
Mount AS, Wheeler AP, Paradkar RP, Snider D (2004) Hemocyte-mediate shell mineralization in the eastern oyster. Science 304:297–300
Moya A, Huisman L, Ball E, Hayward D, Grasso L, Chua C, Woo HN, Gattuso JP, Foret S, Miller DJ (2012) Whole transcriptome analysis of the coral Acropora millepora reveals complex responses to CO2 driven acidification during the initiation of calcification. Mol Ecol 21:2440–2454
Moya A, Huisman L, Foret S, Gattuso JP, Hayward DC, Ball EE, Miller DJ (2015) Rapid acclimation of juvenile corals to CO2-mediated acidification by upregulation of heat shock protein and Bcl-2 genes. Mol Ecol 24(2):438–452
Moya A, Howes EL, Lacoue-Labarthe T, Foret S, Hanna B, Medina M, Munday PL, Ong JS, Yeyssie JL, Torda G, Watson SA, Miller DJ, Bijma J, Gattuso JP (2016) Near-future pH conditions severely impact calcification, metabolism, and the nervous system in the pteropod Heliconoides inflatus. Glob Change Biol 22:3888–3900
Nagai K, Yano M, Morimoto K, Miyamoto H (2007) Tyrosinase localization in mollusc shells. Comp. Biochem. Physiol. B. Biochem Mol Biol 146:207–214
Nikapitiya C, De Zoysa M, Oh C, Lee Y, Ekanayake PM, Whang I, Choi CY, Lee JS, Lee J (2010) Disk abalone (Haliotis discus discus) expresses a novel antistasin-like serine protease inhibitor: molecular cloning and immune response against bacterial infection. Fish Shellfish Immunol 28:661–671
Nilius B, Appendino G, Owsianik G (2012) The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflug Arch Eur J Phy 464:425–458
O’Donnell MJ, Todgham AE, Sewell MA, Hammond LM, Ruggiero K, Fangue NA, Zippay ML, Hofmann GE (2010) Ocean acidification alters skeletogenesis and gene expression in larval sea urchins. Mar Ecol Prog Ser 398:157–171
Palumbi S, Oliver T (2006) Genetic evidence of dispersal limitation and local adaptation in Samoan reef corals: report on ongoing research: March 2006
Pan TCF, Applebaum SL, Manahan DT (2015) Experimental ocean acidification alters the allocation of metabolic energy. Proc Natl Acad Sci 112:4696–4701. https://doi.org/10.1073/pnas.1416967112
Parker LM, Ross PM, O’Connor WA (2011) Populations of the Sydney rock oyster, Saccostrea glomerata, vary in response to ocean acidification. Mar Biol 158:689–697
Patnaik BB, Wang TH, Kang SW, Hwang HJ, Park SY, Park EB, Chung JM, Song DK, Kim C, Kim S, Lee JS, Han YS, Park HS, Lee YS (2016) Sequencing, de novo assembly, and annotation of the transcriptome of the endangered freshwater pearl bivalve, Cristaria plicata, provides novel insights into functional genes and marker discovery. PLoS ONE 11:e0148622. https://doi.org/10.1371/journal.pone.0148622
Pauletto M, Milan M, Moreira R, Novoa B, Figueras A, Babbucci M, Patarnello T, Bargelloni L (2014) Deep transcriptome sequencing of Pecten maximus hemocytes: a genomic resource for bivalve immunology. Fish and Shellfish Immunol 37:154–165
Pespeni MH, Sanford E, Gaylord B, Hill TM, Hosflet JD, Jaris HK, LaVigne M, Lenz EA, Russell AD, Young MK, Palumbi SR (2013) Evolutionary change during experimental ocean acidification. Proc Natl Acad Sci USA 110:6937–6942
Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE (2012) Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 7:e37135. https://doi.org/10.1371/journal.pone.0037135
Powell D, Subramanian S, Suwansaard S, Zhao M, O’Connor W, Raftos D, Elizur A (2018) The genome of the oyster Saccostrea offers insight into the environmental resilience of bivalves. DNA Res 25:655–665
Putri GH, Anders S, Pyl PT, Pimada JE, Zanini F (2022) Analyzing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics. https://doi.org/10.1093/bioinformatics/btac166
Rajan KC, Vengatesen T (2020) Molecular adaptation of molluscan biomineralzation to high CO2 oceans- the known and the unknown. Mar Environ Res 155:104883
Rajan KC, Meng Y, Roberts SB, Vengatesen T (2021) Oyster biomineralization under ocean acidification: from genes to shell. Glob Change Biol 27:3779–3797
Ramajo L, Marba N, Prado L, Peron S, Lardies M, Rodriguez-Navarroo VCA, Lagos NA, Duarte CM (2015) Biomineralization changes with food supply confer juvenile scallops (Agropecten purpuratus) resistance to ocean acidification. Glob Change Biol 22:2025–2037
Ramajo L, Perez-Leon E, Hendriks IE, Marba N, Krause-Jensen D, Sejr MK, Blicher ME, Lagos N, Olsen Y, Duarte CM (2016) Food supply confers calcifiers resistance to ocean acidification. Sci Rep 6:19374. https://doi.org/10.10138/srep19374
Reusch TBH (2013) Climate change in the oceans: evolutionary versus phenotypically plastic responses of marine animals and plants. Evo App 7:104–122
Rochette NC, Rivera-Colón AG, Catchen JM (2019) Stacks 2: analytical methods for paired-end sequencing improve RADseq-based population genomics. Mol Ecol 28(21):4737–4754. https://doi.org/10.1111/mec.15253
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140
Rodolfo-Metalpa R, Lombardi C, Cocito S, Hall-Spencer JM, Gambi MC (2010) Effects of ocean acidification and high temperatures on the bryozoan Myriapora truncata at natural CO2 vents. Mar Ecol 31:447–456
Ronza P, Cao A, Robledo D, Gomez-Tato A, Alvarez-Dios JA, Hasanuzzaman AFM, Quiroga MI, Villalba A, Pardo BG, Martinez P (2018) Long-term affected flat oyster (Ostrea edulis) haemocytes show differential gene expression profiles from naive oysters in response to Bonamia ostreae. Genomics 110:390–398
Saba GK, Goldsmith KA, Cooley SR, Grosse D, Meseck SL (2019) Recommended priorities for research on ecological impacts of ocean and coastal acidification in the U.S. mid-Atlantic. Estuar Coast Shelf Sci 225:106188. https://doi.org/10.1016/j.ecss.2019.04.022
Saco A, Rey-Campos M, Novoa B, Figueras A (2020) Transcriptomic response of mussel gills after a Vibrio splendidus infection demonstrates their role in the immune response. Front Immunol 11:615580. https://doi.org/10.3389/fimmu.2020.615580
Scheufler C, Brinker A, Bourenkkov G, Pegoraro S, Moroder L, Bartunik H (2000) Structure of TPR domain-peptide complexes: critical elements in the assembly of the HSP70-HSP90 multichaperone machine. Cell 101:199–210
Schluter L, Lohbeck KT, Gutowska MA, Groger JP, Riebesell U, Reusch T (2014) Adaptation of a globally important coccolithophore to ocean warming and acidification. Nat Clim Change 4:1024–1030
Schwaner C, Barbosa M, Connors P, Park TJ, de Silva D, Griffith A, Gobbler CJ, Pales Espinosa E, Allam B (2020) Experimental acidification increases susceptibility of mercenaria mercenaria to infection by vibrio species. Mar Environ Res 154:104872. https://doi.org/10.1016/j.marenvres.2019.10487
Schwaner C, Farhat S, Barbosa M, Boutet I, Tanguy A, Pales Espinosa E, Allam B (2022a) Molecular features associated with resilience to ocean acidification in the northern quahog, Mercenaria mercenaria. Mar Biotechnol. https://doi.org/10.1007/s10126-022-10183
Schwaner C, Farhat S, Haley J, Pales Espinosa E, Allam B (2022b) Proteomic and transcriptomic responses enable clams to correct the pH of calcifying fluids and sustain biomineralization in acidified environments. Int J Mol Sci 23:16066. https://doi.org/10.3390/imjs232416066
Schwaner C, Pales Espinosa E, Allam B (2023) RNAi silencing of the biomineralization gene perlucin impars oyster ability to cope with ocean acidification. Int J Mol Sci 2:3661. https://doi.org/10.3390/ijms24043661
Shen M, Di G, Li M, Fu J, Dai Q, Miao X, Huang M, You W, Ke C (2018) Proteomics studies on three larval stages of development and metamorphosis of Babylonia areolata. Sci Rep 8:6269. https://doi.org/10.1093/gbe/evy242
Shi Y, Yu C, Gu Z, Zhan X, Wang Y, Wang A (2013) Characterization of the pearl oyster (Pinctada martensii) mantle transcriptome unravels biomineralization genes. Mar Biotechnol 15:175–187
Shimizu K, Kimura K, Isowa Y, Oshima K, Ishikawa M, Kagi H, Kito K, Hattori M, Chiba S, Endo K (2019) Insights into the evolution of shells and love darts of land snails revealed from their matrix proteins. Genome Biol Evol 11:380–397
Sigle LT, Hillyer JF (2018) Eater and draper are involved in the periostial haemocyte immune response in the mosquito Anopheles gambiae. Insect Mol Biol 27:429–438
Strader ME, Wong JM, Hofmann GE (2020) Ocean acidification promotes broad transcriptomic responses in marine metazoans: a literature survey. Front Zool 17:1–23
Su W, Rong J, Zha S, Yan M, Fang J, Liu G (2018) Ocean acidification affects the cytoskeleton, lysozymes, and nitric oxide of hemocytes: a possible explanation for the hampered phagocytosis in blood clams. Tegillarca Granosa Front Aquat Phys 9:619. https://doi.org/10.3389/fphys.2018.00619
Sunday JM, Calosi P, Dupont S, Munday PL, Stillman JH, Reusch TBH (2014) Evolution in an acidifying ocean. Trends Ecol Evol 29:117–125
Suzuki M, Nagasawa H (2013) Mollusk shell structures and their formation mechanism. Can J Zool 91:349–366
Talmage SC, Gobler CJ (2010) Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. Proc Natl Acad Sci USA 107:17246–17251. https://doi.org/10.1073/pnas.0913804107
Tanio M, Kondo S, Sugio S, Kohno T (2007) Trivalent recognition unit of innate immunity system: crystal structure of trimeric human M-ficolin fibrinogen-like domain. J Biol Chem 282:3889–3895
Thomsen J, Casties I, Pansch C, Körtzinger A, Melzner F (2013) Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: laboratory and field experiments. Glob Change Biol 19:1017–1027
Thomsen J, Haynert K, Wegner KM, Melzner F (2015) Impact of seawater carbonate chemistry on the calcification of marine bivalves. Biogeosciences 12:1543–1571. https://doi.org/10.5194/bg-12-4209-2015
Thomsen J, Stapp LS, Haynert K, Schade H, Danelli M, Lanning G, Melzner F (2017) Naturally acidified habitat selects for ocean acidification-tolerant mussels. Sci Adv 3:e1602411. https://doi.org/10.1126/sciadv.1602411
Todgham AE, Hofmann GE (2009) Transcriptomic response of sea urchin larvae Strongylocentrotus purpuratus to CO2-driven seawater acidification. J Exp Biol 212:2579–2594
Tomanek L, Zuzow MJ, Ivanina AV, Beniash E, Sikolova IM (2011) Proteomic response to elevated pCO2 level in eastern oysters, Crassostrea virginica: evidence for oxidative stress. J Exp Biol 214:1836–1844
Timmins-Schiffman E, O’Donnell MJ, Friedman CS, Roberts SB (2013) Elevated pCO2 causes developmental delay in early larval Pacific oysters, Crassostrea gigas. Mar Biol 160:1973–1982
Timmins-Schiffman E, Coffey WD, Hua W, Nunn BL, Dickinson GH, Roberts SB (2014) Shotgun proteomics reveals physiological response to ocean acidification in Crassostrea gigas. BMC Genomics 15:951. https://doi.org/10.1186/1471-2164-15-951
Trigg SA, Mitchell KR, Thompson RE, Eudeline B, Vadopalas B, Timmins-Schiffman EB, Roberts S (2020) Temporal proteomic profiling reveals insight into critical developmental processes and temperature-influenced physiological response differences in a bivalve mollusc. BMC Genomics 21:723. https://doi.org/10.1101/2020.06.05.137059
Uthicke S, Deshpande NP, Liddy M, Patel F, Lamare M, Wilkins MR (2019) Little evidence of adaptation potential to ocean acidification in sea urchins living in “Future Ocean” conditions at a CO2 vent. Ecol Evol 9:10004–100016
Wage J, Lerebours A, Hardege JD, Rotchell JM (2016) Exposure to low pH induces molecular level changes in the marine worm, Platynereis dumerilii. Ecotoxicol Environ Saf 124:105–110
Waldbusser GG, Salisbury JE (2014) Ocean acidification in the coastal zone from an organism’s perspective: multiple system parameters, frequency domains, and habitats. Ann Rev Mar Sci 6:221–247
Wallace RB, Baumann H, Great JS, Aller RC, Gobbler CJ (2014) Coastal ocean acidification: the other eutrophication problem. Estuar Coast Shelf Sci 148:1–13
Waldbusser GG, Brunner EL, Haley BA, Hales B, Langdon CJ, Prahl FG (2013) A developmental and energetic basis linking larval oyster shell formation to acidification sensitivity. Geophys Res Lett 40(10):2171–2176. https://doi.org/10.1002/grl.50449
Wang N, Lee YH, Lee J (2008) Recombinant perlucin nucleates the growth of calcium carbonate crystals: molecular cloning and characterization of perlucin from disk abalone, Haliotis discus discus. Comp Biochem Physiol B: Biochem 149:354–361. https://doi.org/10.1016/j.cbpb.2007.10.007
Wang Q, Cao R, Ning X, You L, Mu C, Wang C, Wei L, Cong M, Wu H, Zhao J (2016) Effects of ocean acidification on immune response of the Pacific oyster Crassostrea gigas. Fish and Shellfish Immunol 49:24–33
Wang X, Wang M, Wang W, Liu Z, Xu J, Jia Z, Chen H, Qiu L, Lv Z, Wang L, Song L (2020) Transcriptional changes of Pacific oyster Crassostrea gigas reveal essential role of calcium signal pathway in response to CO2-driven acidification. Sci Total Environ 741:140177. https://doi.org/10.1015/j.scitotenv.2020.140177
Wei J, Liu B, Fan S, Li H, Chen M, Zhang B, Su J, Meng Z, Yu D (2017) Differentially expressed immune-related genes in hemocytes of the pearl oyster Pinctada fucata against allograft identified by transcriptome analysis. Fish and Shellfish Immunol 62:247–256
Whitlock MC, Lotterhos KE (2015) Reliable detection of loci responsible for local adaptation: inference of a null model through trimming the distribution of F(ST). Am Nat Supp 1:S24-36
Xiong Y, Hu L, Yan Z, Zhang J, Li H (2018) Transcriptomic analysis of embryo development in the invasive snail Pomacea canaliculata. J Molluscan Stud 84(3):233–239
Yang B, Pu F, Li L, You W, Ke C, Feng D (2017) Functional analysis of a tyrosinase gene involved in early larval shell biogenesis in Crassostrea angulata and its response to ocean acidification. Comp Biochem Physiol B Biochem Mol Biol 206:8–15
Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, Wang J (2012a) The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490:49–54. https://doi.org/10.1038/nature11413
Zhang Z, Miteva MA, Wang L, Alexov E (2012b) Analyzing effects of naturally occurring missense mutations. Comput Math Methods Med 2012:805827. https://doi.org/10.1155/2012/805827
Zhang G, Li L, Meng J, Qi H, Qu T, Xu F, Zhang L (2016) Molecular basis for adaptation of oysters to stressful marine intertidal environments. Annu Rev Anim Bio 4:357–381. https://doi.org/10.1146/annurev-animal-022114-110903
Zhang C, Xue Z, Yu Z, Wang H, Liu Y, Li H, Song L (2020) A tandem-repeat galectin-1 from Apostichopus japonicus with broad PAMP recognition pattern and antibacterial activity. Fish Shellfish Immunol 99:167–175
Zhao X, Shi W, Han Y, Liu S, Guo C, Fu W, Chai X, Liu G (2017) Ocean acidification adversely influences metabolism, extracellular pH and calcification of an economically important marine bivalve, Tegillarca granosa. Mar Environ Res 125:82–89
Zhao X, Han Y, Chen B, Xia B, Qu K, Liu G (2020) CO2-drive ocean acidification weakens mussel shell defense capacity and induces global molecular compensatory responses. Chemosphere 243:125415. https://doi.org/10.1016/j.chemosphere.2019.125415
Acknowledgements
The authors would like to thank Marty Byrnes and Rebecca Resner of the Great Atlantic Shellfish Hatchery for providing access to their hatchery and logistical support. We would also like to thank the Marine Animal Disease Laboratory intern Margot Eckstein for help with bivalve maintenance. In addition, we would also like to thank Dr. Christopher Gobler and Andrew Lundstrom for processing DIC samples.
Funding
This research was funded by the New York Sea Grant (Projects R/XG-24 and R/XG-33 to Bassem Allam and Emmanuelle Pales Espinosa). Additional support was provided by the National Science Foundation (IOS 1656753) and the Atlantic States Marine Fisheries Commission (Contract 19–0802).
Author information
Authors and Affiliations
Contributions
Caroline Schwaner contributed to formal analysis, investigation, methodology, and writing the original draft. Sarah Farhat contributed to formal analysis and manuscript revisions. Isabelle Boutet and Arnaud Tanguy assisted with investigation and methodology. Michelle Barbosa helped with investigation. Denis Grouzdev assisted with formal analysis. Emmanuelle Pales Espinosa helped with methodology, manuscript revisions, and funding. Bassem Allam provided funding, assisted with investigation, conceptualization, project administration, and reviewing and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schwaner, C., Farhat, S., Boutet, I. et al. Combination of RNAseq and RADseq to Identify Physiological and Adaptive Responses to Acidification in the Eastern Oyster (Crassostrea virginica). Mar Biotechnol 25, 997–1019 (2023). https://doi.org/10.1007/s10126-023-10255-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-023-10255-y