Abstract
Mesenchymal stem cells (MSCs) can be differentiated into cardiac, endothelial, and smooth muscle cells. Therefore, MSC-based therapeutic approaches have the potential to deal with the aftermaths of cardiac diseases. However, transplanted stem cells rarely survive in damaged myocardium, proposing that paracrine factors other than trans-differentiation may involve in heart regeneration. Apart from cytokines/growth factors, MSCs secret small, single-membrane organelles named exosomes. The MSC-secreted exosomes are enriched in lipids, proteins, nucleic acids, and microRNA (miRNA). There has been an increasing amount of data that confirmed that MSC-derived exosomes and their active molecule microRNA (miRNAs) regulate signaling pathways involved in heart repair/regeneration. In this review, we systematically present an overview of MSCs, their cardiac differentiation, and the role of MSC-derived exosomes and exosomal miRNAs in heart regeneration. In addition, biological functions regulated by MSC-derived exosomes and exosomal-derived miRNAs in the process of heart regeneration are reviewed.
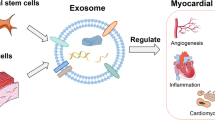
Graphical Abstract


Similar content being viewed by others
Abbreviations
- CVDs:
-
Cardiovascular diseases
- MSCs:
-
Mesenchymal stem cells
- miRNAs:
-
microRNA
- BM-MSCs:
-
Bone marrow MSCs
- AT-MSCs:
-
Adipose tissue-derived MSCs
- UC-MSCs:
-
Umbilical cord MSCs
- ESC-MSCs:
-
Embryonic stem cell-derived MSCs
- iPSC-MSCs:
-
Induced pluripotent stem cell-derived MSCs
- 5-aza:
-
5-Azacytidine
- AA:
-
Ascorbic acid
- BMP2:
-
Bone morphogenetic protein
- DMSO:
-
Dimethylsulfoxide
- EVs:
-
Extracellular vesicles
- ILVs:
-
Intraluminal vesicles
- MVB:
-
Multivesicular body
- ESCRT:
-
Endosomal sorting complexes required for transport
- VEGF:
-
Vascular endothelial growth factor
- MI:
-
Myocardial infarction
- HUVEC:
-
Human umbilical vein endothelial cell
- EGF:
-
Epidermal growth factors
- PDGF:
-
Platelet-derived growth factors
- FGF:
-
Fibroblast-derived growth factors
- TGF:
-
Transforming growth factors
- NF-ĸB:
-
Nuclear factor ĸB
- Ang1:
-
Angiopoietin-1
- Flk1:
-
Fetal liver kinase-1
- Vash1:
-
Vasohibin-1
- EPHB2:
-
Ephrin type-B receptor-2
- NRP2:
-
Neuropilin-2
- SEMA5B:
-
Semaphoring-5B
- NCOA1:
-
Nuclear receptor coactivator-1
- MAZ:
-
MYC-associated zinc finger protein
- ALOX5:
-
Arachidonate 5-Lipoxygenase
- PPM1A:
-
Protein phosphatase-1A
- ROS:
-
Reactive oxygen species
- PI3K:
-
Phosphoinositide 3-kinase
- miRISC:
-
miRNA-induced silencing complex
- SRF:
-
Serum response factor
- MEF2:
-
Myosin enhancer factor-2
- MRF:
-
Myogenic regulatory factor
- CHD:
-
Congenital heart disease
- Tbx5:
-
T-box transcription factor-5
- ANF:
-
Atrial natriuretic factor
- BNP:
-
Brain natriuretic peptide
- PTEN:
-
Phosphatase and tensin homolog
- SOX-6:
-
SRY-box transcription factor-6
- CTGF:
-
Connective tissue growth factor
- HDAC4:
-
Histone deacetylase-4
- Hand:
-
Hand transcription factor-2
- Dll-1:
-
Delta-like 1
- Dab2:
-
Disabled homolog-2
- IGF1:
-
Insulin-like growth factor-1
- CXCR4:
-
Chemokine receptor type 4
- CMs:
-
Cardiomyocytes
- DU145:
-
Human prostate cancer cell line
- hECTs:
-
Human-engineered cardiac tissue
- CSCs:
-
Cardiac stem cells
- HMGA2:
-
High-mobility group AT-Hook 2
- EZH2:
-
Enhancer of zeste homolog 2
- EMT:
-
Epithelial-mesenchymal transition
- LV:
-
Left ventricular
- AMP:
-
Adenosine 5′-monophosphate
- OS:
-
Oxidative stress
- TIMPs:
-
Tissue inhibitor of matrix metalloproteinases
- MMPs:
-
Matrix metalloproteases
References
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol [Internet]. 2020 Dec 12 [cited 2023 Sep 23];76(25):2982. Available from: /pmc/articles/PMC7755038/
Flora GD, Nayak MK. A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Curr Pharm Des. 2019;25(38):4063–84.
Sim HW, Zheng H, Richards AM, Chen RW, Sahlen A, Yeo KK, et al. Beta-blockers and renin-angiotensin system inhibitors in acute myocardial infarction managed with inhospital coronary revascularization. Sci Rep. 10, Article 15184 (2020) [Internet]. 2020 Sep 16 [cited 2023 Sep 23];10(1). Available from: https://doi.org/10.1038/s41598-020-72232-y
Ayuna A, Abidin N. The role of neurohormonal blockers in the primary prevention of acute-, early-, and late-onset anthracycline-induced cardiotoxicity. Egypt Heart J [Internet]. 2020 Dec 1 [cited 2023 Sep 23];72(1):1–7. Available from: https://tehj.springeropen.com/articles/10.1186/s43044-020-00090-0. Accessed 11 Sept 2020.
Gaudino M, Bakaeen FG, Benedetto U, Di Franco A, Fremes S, Glineur D, et al. Arterial grafts for coronary bypass: a critical review after the publication of ART and RADIAL. Circulation [Internet]. 2019 Oct 8 [cited 2023 Sep 23];140(15):1273–84. Available from: https://pubmed.ncbi.nlm.nih.gov/31934782/. Accessed 8 Oct 2019.
Khan MohdS, Khan MohdS. Coronary artery bypass grafting: surgical anastomosis: tips and tricks. Curr Perspect Coron Artery Bypass Grafting [Internet]. 2019 Nov 13 [cited 2023 Sep 23]; Available from: https://www.intechopen.com/chapters/70032. Accessed 13 Nov 2019.
Peng X, Zhou J, Wu XS. New strategies for myocardial infarction treatment. J Cardiol Ther (Hong Kong) [Internet]. 2017 Jun 3 [cited 2023 Sep 23];4(3):664–70. Available from: http://www.ghrnet.org/index.php/jct/article/view/1895/2387. Accessed 3 June 2017.
Gao G, Fan C, Li W, Liang R, Wei C, Chen X, et al. Mesenchymal stem cells: ideal seeds for treating diseases. Hum Cell [Internet]. 2021 Nov 1 [cited 2023 Sep 23];34(6):1585. Available from: /pmc/articles/PMC8284686/
Liu Z, Naveed M, Baig MMFA, Mikrani R, Li C, Saeed M, et al. Therapeutic approach for global myocardial injury using bone marrow-derived mesenchymal stem cells by cardiac support device in rats. Biomed Microdevices [Internet]. 2021 Mar 1 [cited 2023 Sep 23];23(1):1–10. Available from: https://link.springer.com/article/10.1007/s10544-020-00538-9. Accessed 8 Jan 2021.
Gupta S, Sharma A, Archana S, Verma RS. Mesenchymal stem cells for cardiac regeneration: from differentiation to cell delivery. Stem Cell Rev Rep [Internet]. 2021 Oct 1 [cited 2023 Sep 23];17(5):1666–94. Available from: https://link.springer.com/article/10.1007/s12015-021-10168-0. Accessed 17 Oct 2021.
Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells — current trends and future prospective. Biosci Rep [Internet]. 2015 [cited 2023 Sep 23];35(2):191. Available from: /pmc/articles/PMC4413017/
Razzaq SS, Khan I, Naeem N, Salim A, Begum S, Haneef K. Overexpression of GATA binding protein 4 and myocyte enhancer factor 2C induces differentiation of mesenchymal stem cells into cardiac-like cells. World J Stem Cells [Internet]. 2022 Sep 9 [cited 2023 Sep 23];14(9):700. Available from: /pmc/articles/PMC9516467/
Haneef K, Habib R, Naeem N, Salim A. Stem cell factor gene overexpression enhances the fusion potential of rat bone marrow mesenchymal stem cells with cardiomyocytes. Pak J Zool. 2021;53(6):2305.
Haneef K, Ali A, Khan I, Naeem N, Jamall S, Salim A. Role of interleukin-7 in fusion of rat bone marrow mesenchymal stem cells with cardiomyocytes in vitro and improvement of cardiac function in vivo. Cardiovasc Ther [Internet]. 2018 Dec 1 [cited 2023 Sep 23];36(6):e12479. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/1755-5922.12479. Accessed 19 Nov 2018.
Matta A, Nader V, Lebrin M, Gross F, Prats AC, Cussac D, et al. Pre-conditioning methods and novel approaches with mesenchymal stem cells therapy in cardiovascular disease. Cells [Internet]. 2022 May 1 [cited 2023 Sep 23];11(10). Available from: /pmc/articles/PMC9140025/
Tan SJO, Floriano JF, Nicastro L, Emanueli C, Catapano F. Novel applications of mesenchymal stem cell-derived exosomes for myocardial infarction therapeutics. Biomolecules [Internet]. 2020 May 1 [cited 2023 Sep 23];10(5). Available from: https://pubmed.ncbi.nlm.nih.gov/32370160/. Accessed 2 May 2020.
Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther [Internet]. 2022 Dec 1 [cited 2023 Sep 23];7(1). Available from: https://pubmed.ncbi.nlm.nih.gov/35314676/. Accessed 21 Mar 2022.
Khubutiya MS, Vagabov AV, Temnov AA, Sklifas AN. Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models of acute organ injury. Cytotherapy. 2014;16(5):579–85.
Razeghian-Jahromi I, Matta AG, Canitrot R, Zibaeenezhad MJ, Razmkhah M, Safari A, et al. Surfing the clinical trials of mesenchymal stem cell therapy in ischemic cardiomyopathy. Stem Cell Res Ther [Internet]. 2021 Dec 1 [cited 2023 Sep 23];12(1):1–12. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-021-02443-1. Accessed 23 June 2021.
Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020 18:1 [Internet]. 2020 Nov 27 [cited 2023 Sep 23];18(1):1–21. Available from: https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-020-02622-3. Accessed 27 Nov 2020.
Kossl J, Bohacova P, Hermankova B, Javorkova E, Zajicova A, Holan V. Antiapoptotic properties of mesenchymal stem cells in a mouse model of corneal inflammation. Stem Cells Dev. 2021;30(8):418–27.
Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells [Internet]. 2017 Apr 1 [cited 2023 Sep 23];35(4):851–8. Available from: https://doi.org/10.1002/stem.2575
Kore RA, Wang X, Ding Z, Griffin RJ, Tackett AJ, Mehta JL. MSC exosome-mediated cardioprotection in ischemic mouse heart comparative proteomics of infarct and peri-infarct areas. Mol Cell Biochem [Internet]. 2021 Apr 1 [cited 2023 Sep 23];476(4):1691. Available from: /pmc/articles/PMC8186026/
Defo M, Joel M, Yuan J, Wang J, Yan Y, Qian H, et al. MSC: immunoregulatory effects, roles on neutrophils and evolving clinical potentials. Am J Transl Res [Internet]. 2019 [cited 2023 Sep 23];11(6):3890–904. Available from: www.ajtr.org/ISSN:1943-8141/AJTR0096368. Accessed 15 June 2019.
Wu X, Jiang J, Gu Z, Zhang J, Chen Y, Liu X. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Research & Therapy 2020 11:1 [Internet]. 2020 Aug 8 [cited 2023 Sep 23];11(1):1–16. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-020-01855-9. Accessed 8 Aug 2020.
Andrzejewska A, Lukomska B, Janowski M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells [Internet]. 2019 Jul 1 [cited 2023 Sep 23];37(7):855–64. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/stem.3016. Accessed 30 Apr 2019.
Klimczak A, Kozlowska U. Mesenchymal stromal cells and tissue-specific progenitor cells: their role in tissue homeostasis. Stem Cells Int [Internet]. 2016 [cited 2023 Sep 23];2016. Available from: /pmc/articles/PMC4707334/
Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, et al. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem [Internet]. 2003 Aug 15 [cited 2023 Sep 23];89(6):1235–49. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/jcb.10594. Accessed 15 Aug 2003.
Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol [Internet]. 2006 [cited 2023 Sep 23];44(4):215–30. Available from: https://journals.viamedica.pl/folia_histochemica_cytobiologica/article/view/4554. Accessed 16 Jan 2007.
Boiret N, Rapatel C, Veyrat-Masson R, Guillouard L, Guérin JJ, Pigeon P, et al. Characterization of nonexpanded mesenchymal progenitor cells from normal adult human bone marrow. Exp Hematol [Internet]. 2005 Feb [cited 2023 Sep 23];33(2):219–25. Available from: https://pubmed.ncbi.nlm.nih.gov/15676216/. Accessed Feb 2005.
Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, et al. Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med [Internet]. 2019 Sep 1 [cited 2023 Sep 23];13(9):1738–55. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/term.2914.
Lou S, Duan Y, Nie H, Cui X, Du J, Yao Y. Mesenchymal stem cells: biological characteristics and application in disease therapy. Biochimie. 2021;1(185):9–21.
Gonzalez-Vilchis RA, Piedra-Ramirez A, Patiño-Morales CC, Sanchez-Gomez C, Beltran-Vargas NE. Sources, characteristics, and therapeutic applications of mesenchymal cells in tissue engineering. Tissue Eng Regen Med [Internet]. 2022 Apr 1 [cited 2023 Sep 23];19(2):325. Available from: /pmc/articles/PMC8971271/
Kobayashi K, Suzuki K. Mesenchymal stem/stromal cell-based therapy for heart failure — what is the best source? Circ J [Internet]. 2018 [cited 2023 Sep 23];82(9):2222–32. Available from: https://pubmed.ncbi.nlm.nih.gov/30089767/. Accessed 24 Aug 2018.
Goradel NH, Hour FG, Negahdari B, Malekshahi ZV, Hashemzehi M, Masoudifar A, et al. Stem cell therapy: a new therapeutic option for cardiovascular diseases. J Cell Biochem [Internet]. 2018 Jan 1 [cited 2023 Sep 23];119(1):95–104. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/jcb.26169.
Oryan A, Kamali A, Moshirib A, Eslaminejad MB. Role of mesenchymal stem cells in bone regenerative medicine: what is the evidence? Cells Tissues Organs [Internet]. 2017 Aug 1 [cited 2023 Sep 23];204(2):59–83. Available from: https://pubmed.ncbi.nlm.nih.gov/28647733/.
Soltani Amir Hossein Mahdavi L, Soltani L. Role of signaling pathways during cardiomyocyte differentiation of mesenchymal stem cells. Rev Article Cardiol [Internet]. 2022 [cited 2023 Sep 23];147:216–24. Available from: www.karger.com/crd.
Shen H, Wang Y, Zhang Z, Yang J, Hu S, Shen Z. Mesenchymal stem cells for cardiac regenerative therapy: optimization of cell differentiation strategy. Stem Cells Int [Internet]. 2015 [cited 2023 Sep 23];2015. Available from: /pmc/articles/PMC4539177/
Naeem N, Haneef K, Kabir N, Iqbal H, Jamall S, Salim A. DNA methylation inhibitors, 5-azacytidine and zebularine potentiate the transdifferentiation of rat bone marrow mesenchymal stem cells into cardiomyocytes. Cardiovasc Ther [Internet]. 2013 Aug [cited 2023 Sep 23];31(4):201–9. Available from: https://pubmed.ncbi.nlm.nih.gov/22954287/.
Haneef K, Naeem N, Khan I, Iqbal HAA, Kabir N, Jamall S, et al. Conditioned medium enhances the fusion capability of rat bone marrow mesenchymal stem cells and cardiomyocytes. Mol Biol Rep [Internet]. 2014 Jan 28 [cited 2023 Sep 23];41(5):3099–112. Available from: https://link.springer.com/article/10.1007/s11033-014-3170-1. Accessed 28 Jan 2014.
Poomani MS, Mariappan I, Perumal R, Regurajan R, Muthan K, Subramanian V. Mesenchymal stem cell (MSCs) therapy for ischemic heart disease: a promising frontier. Glob Heart [Internet]. 2022 [cited 2023 Sep 23];17(1). Available from: /pmc/articles/PMC8916054/
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol [Internet]. 2013 Feb 2 [cited 2023 Sep 24];200(4):373. Available from: /pmc/articles/PMC3575529/
Battistelli M, Falcieri E. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology (Basel) [Internet]. 2020 Jan 1 [cited 2023 Sep 24];9(1). Available from: https://pubmed.ncbi.nlm.nih.gov/31968627/. Accessed 20 Jan 2020.
Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol [Internet]. 2015 [cited 2023 Sep 24]; Available from: https://doi.org/10.1016/j.tcb.2015.01.004
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology 2007 9:6 [Internet]. 2007 May 7 [cited 2023 Sep 24];9(6):654–9. Available from: https://www.nature.com/articles/ncb1596. Accessed 7 May 2007.
Wei H, Chen Q, Lin L, Sha C, Li T, Liu Y, et al. Regulation of exosome production and cargo sorting. Int J Biol Sci [Internet]. 2021 [cited 2023 Sep 24];17(1):163. Available from: /pmc/articles/PMC7757038/
Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles [Internet]. 2015 [cited 2023 Sep 24];4(2015):1–60. Available from: https://pubmed.ncbi.nlm.nih.gov/25979354/. Accessed 14 May 2015.
Kalluri R. The biology and function of exosomes in cancer. J Clin Invest [Internet]. 2016 Apr 1 [cited 2023 Sep 24];126(4):1208–15. Available from: https://pubmed.ncbi.nlm.nih.gov/27035812/. Accessed 1 Apr 2016.
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol [Internet]. 2019 Jan 1 [cited 2023 Sep 24];21(1):9–17. Available from: https://pubmed.ncbi.nlm.nih.gov/30602770/. Accessed 2 Jan 2019.
Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;1(188):1–11.
Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Communication and Signaling [Internet]. 2021 Dec 1 [cited 2023 Sep 24];19(1). Available from: https://www.researchgate.net/publication/351086259_The_exosome_journey_from_biogenesis_to_uptake_and_intracellular_signalling.
Février B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16(4):415–21.
Khalyfa A, Poroyko VA, Qiao Z, Gileles-Hillel A, Khalyfa AA, Akbarpour M, et al. Exosomes and metabolic function in mice exposed to alternating dark-light cycles mimicking night shift work schedules. Front Physiol [Internet]. 2017 Nov 2 [cited 2023 Sep 24];8(NOV). Available from: https://pubmed.ncbi.nlm.nih.gov/29163218/. Accessed 2 Nov 2017.
Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell & Bioscience 2019 9:1 [Internet]. 2019 Feb 15 [cited 2023 Sep 24];9(1):1–18. Available from: https://cellandbioscience.biomedcentral.com/articles/10.1186/s13578-019-0282-2. Accessed 15 Feb 2019.
Cho KS, Kang SA, Kim SD, Mun SJ, Yu HS, Roh HJ. Dendritic cells and M2 macrophage play an important role in suppression of Th2-mediated inflammation by adipose stem cells-derived extracellular vesicles. Stem Cell Res [Internet]. 2019 Aug 1 [cited 2023 Sep 24];39. Available from: https://pubmed.ncbi.nlm.nih.gov/31344653/. Accessed 12 Jul 2019.
Gyöngyösi M, Blanco J, Marian T, Trón L, Petneházy O, Petrasi Z, et al. Serial noninvasive in vivo positron emission tomographic tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circ Cardiovasc Imaging [Internet]. 2008 [cited 2023 Sep 24];1(2):94. Available from: /pmc/articles/PMC3053595/
McGinley LM, McMahon J, Stocca A, Duffy A, Flynn A, O’Toole D, et al. Mesenchymal stem cell survival in the infarcted heart is enhanced by lentivirus vector-mediated heat shock protein 27 expression. Hum Gene Ther [Internet]. 2013 Oct 1 [cited 2023 Sep 24];24(10):840. Available from: /pmc/articles/PMC3787467/
Kim EH, Kim DH, Kim HR, Kim SY, Kim HH, Bang OY. Stroke serum priming modulates characteristics of mesenchymal stromal cells by controlling the expression miRNA-20a. Cell Transplant [Internet]. 2016 Aug 1 [cited 2023 Sep 24];25(8):1489–99. Available from: https://journals.sagepub.com/doi/10.3727/096368916X690430?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed. Accessed 1 Aug 2016.
Collantes M, Pelacho B, García-Velloso MJ, Gavira JJ, Abizanda G, Palacios I, et al. Non-invasive in vivo imaging of cardiac stem/progenitor cell biodistribution and retention after intracoronary and intramyocardial delivery in a swine model of chronic ischemia reperfusion injury. J Transl Med [Internet]. 2017 Mar 13 [cited 2023 Sep 24];15(1):1–11. Available from: https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-017-1157-0. Accessed 13 Mar 2017.
Kanelidis AJ, Premer C, Lopez J, Balkan W, Hare JM. Route of delivery modulates the efficacy of mesenchymal stem cell therapy for myocardial infarction: a meta-analysis of preclinical studies and clinical trials. Circ Res [Internet]. 2017 Mar 31 [cited 2023 Sep 24];120(7):1139–50. Available from: https://www.ahajournals.org/doi/abs/10.1161/CIRCRESAHA.116.309819.
Sun SJ, Wei R, Li F, Liao SY, Tse HF. Mesenchymal stromal cell-derived exosomes in cardiac regeneration and repair. Stem Cell Rep [Internet]. 2021 Jul 7 [cited 2023 Sep 24];16(7):1662. Available from: /pmc/articles/PMC8282428/
Ma T, Chen Y, Chen Y, Meng Q, Sun J, Shao L, et al. MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int [Internet]. 2018 [cited 2023 Sep 24];2018. Available from: https://pubmed.ncbi.nlm.nih.gov/30271437/. Accessed 9 Sept 2018.
Moghaddam AS, Afshari JT, Esmaeili SA, Saburi E, Joneidi Z, Momtazi-Borojeni AA. Cardioprotective microRNAs: lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis [Internet]. 2019 Jun 1 [cited 2023 Sep 24];285:1–9. Available from: http://www.atherosclerosis-journal.com/article/S0021915019301522/fulltext.
Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor ENE, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res [Internet]. 2013 May [cited 2023 Sep 24];10(3):301–12. Available from: https://pubmed.ncbi.nlm.nih.gov/23399448/.
Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H, et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int [Internet]. 2015 [cited 2023 Sep 24];2015. Available from: https://pubmed.ncbi.nlm.nih.gov/26106430/. Accessed 27 May 2015.
Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J Am Heart Assoc [Internet]. 2016 Jan 1 [cited 2023 Sep 25];5(1). Available from: https://pubmed.ncbi.nlm.nih.gov/26811168/. Accessed 25 Jan 2016.
Cervio E, Barile L, Moccetti T, Vassalli G. Exosomes for intramyocardial intercellular communication. Stem Cells Int [Internet]. 2015 [cited 2023 Sep 24];2015. Available from: /pmc/articles/PMC4454760/
Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) [Internet]. 2014 Apr 1 [cited 2023 Sep 24];92(4):387–97. Available from: https://pubmed.ncbi.nlm.nih.gov/24337504/.
Tirziu D, Simons M. Angiogenesis in the human heart: Gene and cell therapy. Angiogenesis [Internet]. 2005 Dec 25 [cited 2023 Sep 24];8(3):241–51. Available from: https://link.springer.com/article/10.1007/s10456-005-9011-z.
Qu Q, Pang Y, Zhang C, Liu L, Bi Y. Exosomes derived from human umbilical cord mesenchymal stem cells inhibit vein graft intimal hyperplasia and accelerate reendothelialization by enhancing endothelial function. Stem Cell Res Ther [Internet]. 2020 Mar 23 [cited 2023 Sep 24];11(1):1–14. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-020-01639-1. Accessed 23 Mar 2020.
Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y, et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA‐21. Stem Cells Transl Med [Internet]. 2017 Jan 1 [cited 2023 Sep 24];6(1):209. Available from: /pmc/articles/PMC5442741/
Xue C, Shen Y, Li X, Li B, Zhao S, Gu J, et al. Exosomes derived from hypoxia-treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. https://home.liebertpub.com/scd [Internet]. 2018 Apr 1 [cited 2023 Sep 24];27(7):456–65. Available from: https://www.liebertpub.com/doi/10.1089/scd.2017.0296. Accessed 1 Apr 2018.
Wang X, Wang H, Cao J, Ye C. Exosomes from adipose-derived stem cells promotes VEGF-C-dependent lymphangiogenesis by regulating miRNA-132/TGF-β pathway. Cell Physiol Biochem [Internet]. 2018 Sep 1 [cited 2023 Sep 24];49(1):160–71. Available from: https://pubmed.ncbi.nlm.nih.gov/30134228/. Accessed 22 Aug 2018.
Xu H, Wang Z, Liu L, Zhang B, Li B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J Cell Biochem [Internet]. 2020 Mar 1 [cited 2023 Sep 24];121(3):2089–102. Available from: https://pubmed.ncbi.nlm.nih.gov/31736169/.
Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem [Internet]. 2015 Dec 1 [cited 2023 Sep 24];37(6):2415–24. Available from: https://doi.org/10.1159/000438594
Almeria C, Weiss R, Roy M, Tripisciano C, Kasper C, Weber V, et al. Hypoxia conditioned mesenchymal stem cell-derived extracellular vesicles induce increased vascular tube formation in vitro. Front Bioeng Biotechnol [Internet]. 2019 Oct 23 [cited 2023 Sep 24];7:292. Available from: /pmc/articles/PMC6819375/
Bian X, Ma K, Zhang C, Fu X. Therapeutic angiogenesis using stem cell-derived extracellular vesicles: an emerging approach for treatment of ischemic diseases. Stem Cell Research & Therapy 2019 10:1 [Internet]. 2019 Jun 3 [cited 2023 Sep 24];10(1):1–18. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-019-1276-z. Accessed 3 June 2019.
Collino F, Pomatto M, Bruno S, Lindoso RS, Tapparo M, Sicheng W, et al. Exosome and microvesicle-enriched fractions isolated from mesenchymal stem cells by gradient separation showed different molecular signatures and functions on renal tubular epithelial cells. Stem Cell Rev [Internet]. 2017 Apr 1 [cited 2023 Sep 24];13(2):226. Available from: /pmc/articles/PMC5380712/
Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells [Internet]. 2016 Mar 1 [cited 2023 Sep 24];34(3):601. Available from: /pmc/articles/PMC5785927/
Vrijsen KR, Maring JA, Chamuleau SAJ, Verhage V, Mol EA, Deddens JC, et al. Exosomes from cardiomyocyte progenitor cells and mesenchymal stem cells stimulate angiogenesis via EMMPRIN. Adv Healthc Mater [Internet]. 2016 Oct 1 [cited 2023 Sep 24];5(19):2555–65. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/adhm.201600308. Accessed 31 Oct 2016.
Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci [Internet]. 2016 Jun 1 [cited 2023 Sep 24];129(11):2182–9. Available from: https://doi.org/10.1242/jcs.170373
Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res [Internet]. 2012 Feb 3 [cited 2023 Sep 24];11(2):839–49. Available from: https://pubs.acs.org/doi/abs/10.1021/pr200682z.
Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM, et al. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep [Internet]. 2018 Dec 1 [cited 2023 Sep 24];8(1). Available from: /pmc/articles/PMC5780426/
Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med [Internet]. 2011 Sep 9 [cited 2023 Sep 24];51(5):978. Available from: /pmc/articles/PMC3156326/
Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A [Internet]. 2011 Dec 6 [cited 2023 Sep 24];108(49):19725–30. Available from: /pmc/articles/PMC3241791/
Fang L, Moore XL, Dart AM, Wang LM. Systemic inflammatory response following acute myocardial infarction. J Geriatr Cardiol [Internet]. 2015 [cited 2023 Sep 24];12(3):305. Available from: /pmc/articles/PMC4460175/
Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther [Internet]. 2018 Jun 1 [cited 2023 Sep 24];186:73. Available from: /pmc/articles/PMC5981007/
Shao L, Shen Y, Ren C, Kobayashi S, Asahara T, Yang J. Inflammation in myocardial infarction: roles of mesenchymal stem cells and their secretome. Cell Death Discov. 2022;8(1):452.
French BA, Kramer CM. Mechanisms of postinfarct left ventricular remodeling. Drug Discov Today Dis Mech. 2007;4(3):185–96.
Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation [Internet]. 2013 Jul 7 [cited 2023 Sep 24];128(4):388. Available from: /pmc/articles/PMC3801217/
Zhang B, Yin Y, Lai RC, Tan SS, Choo ABH, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev [Internet]. 2014 Jun 1 [cited 2023 Sep 24];23(11):1233–44. Available from: https://pubmed.ncbi.nlm.nih.gov/24367916/.
Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, et al. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci [Internet]. 2017 Jul 6 [cited 2023 Sep 24];18(7). Available from: https://pubmed.ncbi.nlm.nih.gov/28684664/
Fattore A Del, Luciano R, Pascucci L, Goffredo BM, Giorda E, Scapaticci M, et al. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant [Internet]. 2015 [cited 2023 Sep 24];24(12):2615–27. Available from: https://pubmed.ncbi.nlm.nih.gov/25695896/. Accessed 1 Dec 2015.
Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147(1–2):47–54.
Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–72.
Di NM, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43.
Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nature Reviews Immunology 2012 12:5 [Internet]. 2012 Apr 25 [cited 2023 Sep 24];12(5):383–96. Available from: https://www.nature.com/articles/nri3209. Accessed 25 Apr 2012.
Lv K, Li Q, Zhang L, Wang Y, Zhong Z, Zhao J, et al. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics [Internet]. 2019 Oct 11 [cited 2023 Sep 24];9(24):7403–16. Available from: http://www.thno.org://creativecommons.org/licenses/by/4.0/
Mao Q, Liang XL, Zhang CL, Pang YH, Lu YX. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138–5p/Sirt1 axis. Stem Cell Res Ther [Internet]. 2019 Dec 17 [cited 2023 Sep 24];10(1):1–14. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-019-1522-4. Accessed 17 Dec 2019.
Sun X, Shan A, Wei Z, Xu B. Intravenous mesenchymal stem cell-derived exosomes ameliorate myocardial inflammation in the dilated cardiomyopathy. Biochem Biophys Res Commun. 2018;503(4):2611–8.
Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J, et al. Engineered exosomes with ischemic myocardium‐targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc [Internet]. 2018 Aug 7 [cited 2023 Sep 24];7(15). Available from: https://www.ahajournals.org/doi/abs/10.1161/JAHA.118.008737.
Shao L, Zhang Y, Lan B, Wang J, Zhang Z, Zhang L, et al. MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. Biomed Res Int [Internet]. 2017 [cited 2023 Sep 24];2017. Available from: https://pubmed.ncbi.nlm.nih.gov/28203568/. Accessed 22 Jan 2017.
Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res [Internet]. 2019 Jun 1 [cited 2023 Sep 24];115(7):1205–16. Available from: https://doi.org/10.1093/cvr/cvz040
Martínez HR, Molina-Lopez JF, González-Garza MT, Moreno-Cuevas JE, Caro-Osorio E, Gil-Valadez A, et al. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant [Internet]. 2013 [cited 2023 Sep 24];22(2):1899–907. Available from: https://pubmed.ncbi.nlm.nih.gov/23433427/.
TIAN XF, CUI MX, YANG SW, ZHOU YJ, HU DY. Cell death, dysglycemia and myocardial infarction. Biomed Rep [Internet]. 2013 May [cited 2023 Sep 24];1(3):341. Available from: /pmc/articles/PMC3917009/
He JG, Li HR, Han JX, Li BB, Yan D, Li HY, et al. GATA-4-expressing mouse bone marrow mesenchymal stem cells improve cardiac function after myocardial infarction via secreted exosomes. Sci Rep [Internet]. 2018 Dec 1 [cited 2023 Sep 24];8(1):9047. Available from: /pmc/articles/PMC5998064/
Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, et al. Exosomes derived from Akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl Med [Internet]. 2017 Jan 1 [cited 2023 Sep 24];6(1):51–9. Available from: https://pubmed.ncbi.nlm.nih.gov/28170176/.
Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One [Internet]. 2014 Feb 18 [cited 2023 Sep 24];9(2). Available from: https://pubmed.ncbi.nlm.nih.gov/24558412/. Accessed 18 Feb 2014.
Liu X, Li X, Zhu W, Zhang Y, Hong Y, Liang X, et al. Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. J Cell Physiol [Internet]. 2020 Nov 1 [cited 2023 Sep 24];235(11):8010–22. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/jcp.29456.
Ni J, Liu X, Yin Y, Zhang P, Xu YW, Liu Z. Exosomes derived from TIMP2-modified human umbilical cord mesenchymal stem cells enhance the repair effect in rat model with myocardial infarction possibly by the Akt/Sfrp2 pathway. Oxid Med Cell Longev [Internet]. 2019 [cited 2023 Sep 24];2019. Available from: https://pubmed.ncbi.nlm.nih.gov/31182988/. Accessed 28 Apr 2019.
Xiao C, Wang K, Xu Y, Hu H, Zhang N, Wang Y, et al. Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of miR-125b. Circ Res [Internet]. 2018 [cited 2023 Sep 24];123(5):564–78. Available from: https://www.ahajournals.org/doi/abs/https://doi.org/10.1161/CIRCRESAHA.118.312758. Accessed 19 June 2018.
Yu B, Gong M, Wang Y, Millard RW, Pasha Z, Yang Y, et al. Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS One [Internet]. 2013 Aug 28 [cited 2023 Sep 24];8(8). Available from: https://pubmed.ncbi.nlm.nih.gov/24015301/. Accessed 28 Aug 2013.
Luther KM, Haar L, McGuinness M, Wang Y, Lynch IV TL, Phan A, et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J Mol Cell Cardiol [Internet]. 2018 Jun 1 [cited 2023 Sep 24];119:125–37. Available from: https://pubmed.ncbi.nlm.nih.gov/29698635/.
Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res [Internet]. 1988 [cited 2023 Sept 24];62(4):757–65. Available from: https://pubmed.ncbi.nlm.nih.gov/2964945/.
van den Borne SWM, Isobe S, Verjans JW, Petrov A, Lovhaug D, Li P, et al. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J Am Coll Cardiol. 2008;52(24):2017–28.
De Haas HJ, Arbustini E, Fuster V, Kramer CM, Narula J. Molecular imaging of the cardiac extracellular matrix. Circ Res [Internet]. 2014 Feb 28 [cited 2023 Sept 25];114(5):903–15. Available from: https://www.ahajournals.org/doi/abs/10.1161/circresaha.113.302680. Accessed 28 Feb 2014.
Shao L, Zhang Y, Lan B, Wang J, Zhang Z, Zhang L, et al. MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. Biomed Res Int [Internet]. 2017 [cited 2023 Sep 25];2017. Available from: https://pubmed.ncbi.nlm.nih.gov/28203568/. Accessed 22 Jan 2017.
Jiao W, Hao J, Xie Y, Meng M, Gao W. EZH2 mitigates the cardioprotective effects of mesenchymal stem cell-secreted exosomes against infarction via HMGA2-mediated PI3K/AKT signaling. BMC Cardiovasc Disord [Internet]. 2022 Dec 1 [cited 2023 Sep 25];22(1):1–13. Available from: https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/s12872-022-02533-9.
Chen F, Li X, Zhao J, Geng J, Xie J, Xu B. Bone marrow mesenchymal stem cell-derived exosomes attenuate cardiac hypertrophy and fibrosis in pressure overload induced remodeling. In Vitro Cell Dev Biol Anim [Internet]. 2020 Aug 1 [cited 2023 Sep 25];56(7):567–76. Available from: https://pubmed.ncbi.nlm.nih.gov/32748023/. Accessed 3 Aug 2020.
Lin Y, Zhang F, Lian XF, Peng WQ, Yin CY. Mesenchymal stem cell-derived exosomes improve diabetes mellitus-induced myocardial injury and fibrosis via inhibition of TGF-Î21/Smad2 signaling pathway. Cell Mol Biol [Internet]. 2019 Sep 30 [cited 2023 Sep 25];65(7):123–6. Available from: https://www.cellmolbiol.org/index.php/CMB/article/view/3059. Accessed 14 Oct 2019.
Wen Z, Zheng S, Zhou C, Wang J, Wang T. Repair mechanisms of bone marrow mesenchymal stem cells in myocardial infarction. J Cell Mol Med [Internet]. 2011 May 1 [cited 2023 Sep 25];15(5):1032–43. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1582-4934.2010.01255.x.
Liu Z, Xu Y, Wan Y, Gao J, Chu Y, Li J. Exosomes from adipose-derived mesenchymal stem cells prevent cardiomyocyte apoptosis induced by oxidative stress. Cell Death Discov [Internet]. 2019 Dec 1 [cited 2023 Sep 25];5(1). Available from: /pmc/articles/PMC6425027/
O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9(AUG):388354.
Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nature Reviews Genetics 2011 12:2 [Internet]. 2011 Jan 18 [cited 2023 Sep 24];12(2):99–110. Available from: https://www.nature.com/articles/nrg2936. Accessed 18 Jan 2011.
Zhang J, Zhou W, Liu Y, Liu T, Li C, Wang L. Oncogenic role of microRNA-532–5p in human colorectal cancer via targeting of the 5′UTR of RUNX3. Oncol Lett [Internet]. 2018 May 1 [cited 2023 Sep 24];15(5):7215–20. Available from: https://pubmed.ncbi.nlm.nih.gov/29849790/. Accessed 8 Mar 2018.
Dharap A, Pokrzywa C, Murali S, Pandi G, Vemuganti R. MicroRNA miR-324–3p induces promoter-mediated expression of RelA gene. PLoS One [Internet]. 2013 Nov 12 [cited 2023 Sep 24];8(11):79467. Available from: /pmc/articles/PMC3827167/
Wang J, Chen J, Sen S. MicroRNA as biomarkers and diagnostics. J Cell Physiol [Internet]. 2016 Jan 1 [cited 2023 Sep 24];231(1):25–30. Available from: https://pubmed.ncbi.nlm.nih.gov/26031493/.
Pang JKS, Phua QH, Soh BS. Applications of miRNAs in cardiac development, disease progression and regeneration. Stem Cell Research & Therapy 2019 10:1 [Internet]. 2019 Nov 21 [cited 2023 Sep 24];10(1):1–11. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-019-1451-2. Accessed 21 Nov 2019.
Ouyang Z, Wei K. miRNA in cardiac development and regeneration. Cell Regen [Internet]. 2021 Dec 1 [cited 2023 Sep 24];10(1). Available from: https://pubmed.ncbi.nlm.nih.gov/34060005/.
Izarra A, Moscoso I, Cañón S, Carreiro C, Fondevila D, Martín-Caballero J, et al. miRNA-1 and miRNA-133a are involved in early commitment of pluripotent stem cells and demonstrate antagonistic roles in the regulation of cardiac differentiation. J Tissue Eng Regen Med [Internet]. 2017 Mar 1 [cited 2023 Sep 24];11(3):787–99. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/term.1977.
Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genom Proteomics Bioinforma [Internet]. 2015 Feb 1 [cited 2023 Sep 24];13(1):17–24. Available from: https://pubmed.ncbi.nlm.nih.gov/25724326/. Accessed 24 Feb 2015.
Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics [Internet]. 2018 Nov 29 [cited 2023 Sep 24];8(22):6163–77. Available from: http://www.thno.org. Accessed 29 Nov 2018.
Mayourian J, Ceholski DK, Gorski PA, Mathiyalagan P, Murphy JF, Salazar SI, et al. Exosomal microRNA-21–5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circ Res [Internet]. 2018 Mar 30 [cited 2023 Sep 24];122(7):933–44. Available from: https://pubmed.ncbi.nlm.nih.gov/29449318/. Accessed 15 Feb 2018.
Ma T, Chen Y, Chen Y, Meng Q, Sun J, Shao L, et al. MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int [Internet]. 2018 [cited 2023 Sep 24];2018. Available from: /pmc/articles/PMC6151206/
Gollmann-Tepekoylu C, Polzl L, Graber M, Hirsch J, Nagele F, Lobenwein D, et al. miR-19a-3p containing exosomes improve function of ischaemic myocardium upon shock wave therapy. Cardiovasc Res [Internet]. 2020 [cited 2023 Sep 24];116(6):1226–36. Available from: https://pubmed.ncbi.nlm.nih.gov/31410448/.
Gao F, Kataoka M, Liu N, Liang T, Huang ZP, Gu F, et al. Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nat Commun [Internet]. 2019 Dec 1 [cited 2023 Sep 24];10(1). Available from: https://pubmed.ncbi.nlm.nih.gov/30996254/.
Gou L, Xue C, Tang X, Fang Z. Inhibition of Exo-miR-19a-3p derived from cardiomyocytes promotes angiogenesis and improves heart function in mice with myocardial infarction via targeting HIF-1α. Aging (Albany NY) [Internet]. 2020 Dec 12 [cited 2023 Sep 24];12(23):23609. Available from: /pmc/articles/PMC7762502/
Wang N, Chen C, Yang D, Liao Q, Luo H, Wang X, et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2017;1863(8):2085–92.
Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from miR-126-overexpressing ADSCs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem [Internet]. 2018 Jan 12 [cited 2023 Sep 24];44(6):2105–16. Available from: https://doi.org/10.1159/000485949
Wei Z, Qiao S, Zhao J, Yihai L, Qiaoling L, Zhonghai W, et al. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci [Internet]. 2019 Sep 1 [cited 2023 Sep 24];232. Available from: https://pubmed.ncbi.nlm.nih.gov/31278944/.
Domenis R, Cifù A, Quaglia S, Pistis C, Moretti M, Vicario A, et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci Rep [Internet]. 2018 Dec 1 [cited 2023 Sep 24];8(1). Available from: https://pubmed.ncbi.nlm.nih.gov/30190615/.
Peng Y, Zhao JL, Peng ZY, Xu WF, Yu GL. Exosomal miR-25–3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis [Internet]. 2020 May 1 [cited 2023 Sep 24];11(5). Available from: https://pubmed.ncbi.nlm.nih.gov/32371945/.
Shen D, He Z. Mesenchymal stem cell-derived exosomes regulate the polarization and inflammatory response of macrophages via miR-21–5p to promote repair after myocardial reperfusion injury. Ann Transl Med [Internet]. 2021 Aug [cited 2023 Sep 24];9(16):1323–1323. Available from: https://pubmed.ncbi.nlm.nih.gov/34532460/. Accessed 28 Aug 2021.
Chen Y, Zhao Y, Chen W, Xie L, Zhao ZA, Yang J, et al. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res Ther [Internet]. 2017 Nov 25 [cited 2023 Sep 24];8(1):1–11. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-017-0722-z. Accessed 25 Nov 2017.
Wen Z, Mai Z, Zhu X, Wu T, Chen Y, Geng D, et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther [Internet]. 2020 Jan 23 [cited 2023 Sep 24];11(1):1–17. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-020-1563-8. Accessed 23 Jan 2020.
Sun XH, Wang X, Zhang Y, Hui J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486–5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res [Internet]. 2019 May 1 [cited 2023 Sep 24];177:23–32. Available from: http://www.thrombosisresearch.com/article/S0049384819300337/fulltext.
Cui X, He Z, Liang Z, Chen Z, Wang H, Zhang J. Exosomes from adipose-derived mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through Wnt/β-catenin signaling pathway. J Cardiovasc Pharmacol [Internet]. 2017 Oct 1 [cited 2023 Sep 24];70(4):225–31. Available from: https://pubmed.ncbi.nlm.nih.gov/28582278/.
Shi B, Wang Y, Zhao R, Long X, Deng W, Wang Z. Bone marrow mesenchymal stem cell-derived exosomal miR-21 protects C-kit+ cardiac stem cells from oxidative injury through the PTEN/PI3K/Akt axis. PLoS One [Internet]. 2018 Feb 1 [cited 2023 Sep 24];13(2). Available from: https://pubmed.ncbi.nlm.nih.gov/29444190/.
Zhang CS, Shao K, Liu CW, Li CJ, Yu BT. Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. Eur Rev Med Pharmacol Sci [Internet]. 2019 [cited 2023 Sep 24];23(15):6691–9. Available from: https://pubmed.ncbi.nlm.nih.gov/31378912/. Accessed Aug 2019.
Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol [Internet]. 2015 Mar 3 [cited 2023 Sep 24];182(C):349. Available from: /pmc/articles/PMC4382384/
Zhu W, Sun L, Zhao P, Liu Y, Zhang J, Zhang Y, et al. Macrophage migration inhibitory factor facilitates the therapeutic efficacy of mesenchymal stem cells derived exosomes in acute myocardial infarction through upregulating miR-133a-3p. J Nanobiotechnology [Internet]. 2021 Dec 1 [cited 2023 Sep 24];19(1):1–16. Available from: https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-021-00808-5. Accessed 27 Feb 2021.
Fu DL, Jiang H, Li CY, Gao T, Liu MR, Li HW. MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. Eur Rev Med Pharmacol Sci. 2020;24(19):10107–17.
Ou H, Teng H, Qin Y, Luo X, Yang P, Zhang W, et al. Extracellular vesicles derived from microRNA-150–5p-overexpressing mesenchymal stem cells protect rat hearts against ischemia/reperfusion. Aging (Albany NY) [Internet]. 2020 Jul 7 [cited 2023 Sep 24];12(13):12669. Available from: /pmc/articles/PMC7377831/
Yang C, Luo M, Chen Y, You M, Chen Q. MicroRNAs as important regulators mediate the multiple differentiation of mesenchymal stromal cells. Front Cell Dev Biol. 2021;7(9):619842.
Fakhry M, Hamade E, Badran B, Buchet R, Magne D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J Stem Cells [Internet]. 2013 [cited 2023 Sep 24];5(4):136. Available from: https://pubmed.ncbi.nlm.nih.gov/24179602/. Accessed 26 Oct 2013.
Lin X, Wu L, Zhang Z, Yang R, Guan Q, Hou X, et al. MiR-335–5p promotes chondrogenesis in mouse mesenchymal stem cells and is regulated through two positive feedback loops. J Bone Miner Res [Internet]. 2014 [cited 2023 Sep 24];29(7):1575–85. Available from: https://pubmed.ncbi.nlm.nih.gov/24347469/.
Shan ZX, Lin QX, Yu XY, Deng CY, Li XH, Zhang XC, et al. MicroRNAs can be expressed in cardiomyocyte-like cells differentiated from human mesenchymal stem cells. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27(12):1813–6.
Collino F, Bruno S, Deregibus MC, Tetta C, Camussi G. MicroRNAs and mesenchymal stem cells. Vitam Horm. 2011;1(87):291–320.
Naguibneva I, Ameyar-Zazoua M, Polesskaya A, Ait-Si-Ali S, Groisman R, Souidi M, et al. The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nat Cell Biol [Internet]. 2006 Mar [cited 2023 Sep 24];8(3):278–84. Available from: https://pubmed.ncbi.nlm.nih.gov/16489342/.
Rongrong W, Xiaozhou M, Xinyang H, Yaping W, Chunsheng X, Jian-an W. e0205 MicroRNA regulation of cardiomyocyte lineages from bone marrowderived mesenchymal stem cells. Heart [Internet]. 2010 Oct 1 [cited 2023 Sep 24];96(Suppl 3):A66–A66. Available from: https://heart.bmj.com/content/96/Suppl_3/A66.1
Lee SY, Ham O, Cha MJ, Song BW, Choi E, Kim IK, et al. The promotion of cardiogenic differentiation of hMSCs by targeting epidermal growth factor receptor using microRNA-133a. Biomaterials [Internet]. 2013 Jan [cited 2023 Sep 24];34(1):92–9. Available from: https://pubmed.ncbi.nlm.nih.gov/23069713/.
Dakhlallah D, Zhang J, Yu L, Marsh CB, Angelos MG, Khan M. MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart. J Cardiovasc Pharmacol [Internet]. 2015 [cited 2023 Sep 24];65(3):241. Available from: /pmc/articles/PMC4452997/
Lee SW, Won JY, Kim WJ, Lee J, Kim KH, Youn SW, et al. Snail as a potential target molecule in cardiac fibrosis: paracrine action of endothelial cells on fibroblasts through Snail and CTGF axis. Mol Ther [Internet]. 2013 [cited 2023 Sep 24];21(9):1767. Available from: /pmc/articles/PMC3953993/
Li B, Meng X, Zhang L. microRNAs and cardiac stem cells in heart development and disease. Drug Discov Today [Internet]. 2019 Jan 1 [cited 2023 Sep 24];24(1):233. Available from: /pmc/articles/PMC6261710/
Huang F, Li ML, Fang ZF, Hu XQ, Liu QM, Liu ZJ, et al. Overexpression of microRNA-1 improves the efficacy of mesenchymal stem cell transplantation after myocardial infarction. Cardiology [Internet]. 2013 May 1 [cited 2023 Sep 24];125(1):18–30. Available from: https://doi.org/10.1159/000347081
Zhao XL, Yang B, Ma LN, Dong YH. MicroRNA-1 effectively induces differentiation of myocardial cells from mouse bone marrow mesenchymal stem cells. Artif Cells Nanomed Biotechnol [Internet]. 2016 Oct 2 [cited 2023 Sep 24];44(7):1665–70. Available from: https://pubmed.ncbi.nlm.nih.gov/26376009/.
Lu M, Xu L, Wang M, Guo T, Luo F, Su N, et al. miR-149 promotes the myocardial differentiation of mouse bone marrow stem cells by targeting Dab2. Mol Med Rep [Internet]. 2018 Jun 1 [cited 2023 Sep 24];17(6):8502–9. Available from: https://pubmed.ncbi.nlm.nih.gov/29693140/.
Finkielstein C V., Capelluto DGS. Disabled-2: a modular scaffold protein with multifaceted functions in signaling. Bioessays [Internet]. 2016 Jul 1 [cited 2023 Sep 24];38 Suppl 1:S45–55. Available from: https://pubmed.ncbi.nlm.nih.gov/27417122/. Accessed 15 Jul 2016.
Hofsteen P, Robitaille AM, Chapman DP, Moon RT, Murry CE. Quantitative proteomics identify DAB2 as a cardiac developmental regulator that inhibits WNT/β-catenin signaling. Proc Natl Acad Sci U S A [Internet]. 2016 Jan 26 [cited 2023 Sep 24];113(4):1002–7. Available from: https://pubmed.ncbi.nlm.nih.gov/26755607/.
Chen JJ, Zhou SH. Mesenchymal stem cells overexpressing miR-126 enhance ischemic angiogenesis via the AKT/ERK-related pathway. Cardiol J [Internet]. 2011 [cited 2023 Sep 24];18(6):675–81. Available from: https://www.researchgate.net/publication/51825545_Mesenchymal_stem_cells_overexpressing_MiR-126_enhance_ischemic_angiogenesis_via_the_AKTERK-related_pathway.
Huang F, Zhu X, Hu XQ, Fang ZF, Tang L, Lu XL, et al. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int J Mol Med [Internet]. 2013 Feb [cited 2023 Sep 24];31(2):484–92. Available from: https://pubmed.ncbi.nlm.nih.gov/23229021/.
Iekushi K, Seeger F, Assmus B, Zeiher AM, Dimmeler S. Regulation of cardiac microRNAs by bone marrow mononuclear cell therapy in myocardial infarction. Circulation [Internet]. 2012 Apr 10 [cited 2023 Sep 24];125(14):1765–73. Available from: https://pubmed.ncbi.nlm.nih.gov/22403243/.
Tano N, Kim HW, Ashraf M. microRNA-150 regulates mobilization and migration of bone marrow-derived mononuclear cells by targeting Cxcr4. PLoS One [Internet]. 2011 [cited 2023 Sep 24];6(10):e23114. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0023114.
Chen C, Chen T, Li Y, Xu Y. miR-19a/19b improves the therapeutic potential of mesenchymal stem cells in a mouse model of myocardial infarction. Gene Ther 2020 28:1 [Internet]. 2020 Jan 22 [cited 2023 Sep 24];28(1):29–37. Available from: https://www.nature.com/articles/s41434-020-0122-3. Accessed 22 Jan 2020.
Funding
This study was financially supported by Higher Education, Commission (HEC), Pakistan, scholarship for Ph.D. studies to Akbar N. (No. 121-FBS3-003) and Razzaq S. S. (No. 520–148390-2BS6-011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
Not applicable.
Conflict of Interest
The author(s) declared no potential conflicts of interest for the research, authorship, and/or publication of this article.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akbar, N., Razzaq, S.S., Salim, A. et al. Mesenchymal Stem Cell-Derived Exosomes and Their MicroRNAs in Heart Repair and Regeneration. J. of Cardiovasc. Trans. Res. (2023). https://doi.org/10.1007/s12265-023-10449-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12265-023-10449-8