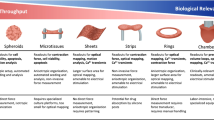
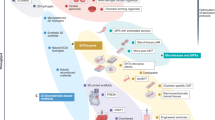
Abstract
Cardiovascular disease (CVD) caused by anti-cancer drug-induced cardiotoxicity is now the second leading cause of mortality among cancer survivors. It is necessary to establish efficient in vitro models for early predicting the potential cardiotoxicity of anti-cancer drugs, as well as for screening drugs that would alleviate cardiotoxicity during and post treatment. Human induced pluripotent stem cells (hiPSCs) have opened up new avenues in cardio-oncology. With the breakthrough of tissue engineering technology, a variety of hiPSC-derived cardiac microtissues or organoids have been recently reported, which have shown enormous potential in studying cardiotoxicity. Moreover, using hiPSC-derived heart-on-chip for studying cardiotoxicity has provided novel insights into the underlying mechanisms. Herein, we summarize different types of anti-cancer drug-induced cardiotoxicities and present an extensive overview on the applications of hiPSC-derived cardiac microtissues, cardiac organoids, and heart-on-chips in cardiotoxicity. Finally, we highlight clinical and translational challenges around hiPSC-derived cardiac microtissues/organoids/heart-on chips and their applications in anti-cancer drug-induced cardiotoxicity.
Graphical abstract
• Anti-cancer drug-induced cardiotoxicities represent pressing challenges for cancer treatments, and cardiovascular disease is the second leading cause of mortality among cancer survivors.
• Newly reported in vitro models such as hiPSC-derived cardiac microtissues/organoids/chips show enormous potential for studying cardio-oncology.
• Emerging evidence supports that hiPSC-derived cardiac organoids and heart-on-chip are promising in vitro platforms for predicting and minimizing anti-cancer drug-induced cardiotoxicity.






Similar content being viewed by others
Data availability
Searches of PubMed and references from relevant articles using the following keywords, alone or in combination, yielded the data for this review: pluripotent stem cells derived cardiomyocytes, ips, cardiac 3D constructs, cardiac organoid, cardioids, engineered heart tissue, heart-on-chip, cancer, cardiotoxicity, toxicity, cardiovascular diseases. Only articles written in English were considered. Articles about novel technologies should be published from 2016 to 2022.
Abbreviations
- ACS:
-
Acute coronary syndrome
- AF:
-
Atrial fibrillation
- AI:
-
Artificial Intelligence
- AMP:
-
Spike amplitude
- ATE:
-
Arterial thromboembolic events
- BNP:
-
B-type natriuretic peptide
- BP:
-
Beating period
- CaMKII:
-
Calmodulin-dependent protein kinase II
- CO:
-
Cardiac organoids
- COUP-TFII:
-
Chick ovalbumin upstream promoter transcription factor II
- cTnT:
-
Cardiac specific troponin T
- CVD:
-
Cardiovascular disease
- DIC:
-
Doxorubicin-induced cardiotoxicity
- ECM:
-
Extracellular matrix
- ESC:
-
Embryonic stem cells
- ECT:
-
Engineered cardiac tissues
- FACS:
-
Fluorescence-activated cell sorting
- FPD:
-
Field potential duration
- HF:
-
Heart failure
- hiPSCs:
-
Human induced pluripotent stem cells
- hiPSC-CMs:
-
Human induced pluripotent stem cell–derived cardiomyocytes
- IRI:
-
Ischemia-reperfusion injury
- LVD:
-
Left ventricular dysfunction
- MI:
-
Myocardial infraction
- MLC2V:
-
Myosin light chain (MLC) 2 V
- mPTP:
-
Mitochondrial permeability transition pore
- NT-proBNP:
-
N-terminal pro B-type natriuretic peptide
- RA:
-
Retinoic acid
- ROS:
-
Reactive oxygen species
- SAN:
-
Sinoatrial node
- SIRPA:
-
Signal-regulatory protein alpha
- VCAM1:
-
Vascular cell adhesion molecule 1
- VTE:
-
Venous thromboembolic events
References
Abecasis B, Canhão PGM, Almeida HV, Calmeiro T, Fortunato E, Gomes-Alves P, Serra M, Alves PM. Toward a microencapsulated 3D hiPSC-derived in vitro cardiac microtissue for recapitulation of human heart microenvironment features. Front Bioeng Biotechnol. 2020;8:580744. https://doi.org/10.3389/fbioe.2020.580744.
Ahmad FB, Anderson RN. The leading causes of death in the US for 2020. JAMA. 2021;325:1829–30. https://doi.org/10.1001/jama.2021.5469.
Anderson D, Self T, Mellor IR, Goh G, Hill SJ, Denning C. Transgenic enrichment of cardiomyocytes from human embryonic stem cells. Mol Ther. 2007;15:2027–36. https://doi.org/10.1038/sj.mt.6300303.
Arai K, Murata D, Takao S, Nakayama K. Fabrication of cardiac constructs using bio-3D printer. Methods Molec Biol. 2021;2320:53–63. https://doi.org/10.1007/978-1-0716-1484-6_6.
Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism: Clin Exp. 2019;92:121–35. https://doi.org/10.1016/j.metabol.2018.11.001.
Beck TC, Arhontoulis DC, Morningstar JE, Hyams N, Stoddard A, Springs K, Mukherjee R, Helke K, Guo L, Moore K, et al. Cellular and molecular mechanisms of MEK1 inhibitor-induced cardiotoxicity. JACC Cardiooncology. 2022;4:535–48. https://doi.org/10.1016/j.jaccao.2022.07.009.
Bergom C, Bradley JA, Ng AK, Samson P, Robinson C, Lopez-Mattei J, Mitchell JD. Past, present, and future of radiation-induced cardiotoxicity: refinements in targeting, surveillance, and risk stratification. JACC Cardiooncology. 2021;3:343–59. https://doi.org/10.1016/j.jaccao.2021.06.007.
Birket MJ, Ribeiro MC, Verkerk AO, Ward D, Leitoguinho AR, den Hartogh SC, Orlova VV, Devalla HD, Schwach V, Bellin M, et al. Expansion and patterning of cardiovascular progenitors derived from human pluripotent stem cells. Nat Biotechnol. 2015;33:970–9. https://doi.org/10.1038/nbt.3271.
Bizy A, Guerrero-Serna G, Hu B, Ponce-Balbuena D, Willis BC, Zarzoso M, Ramirez RJ, Sener MF, Mundada LV, Klos M, et al. Myosin light chain 2-based selection of human iPSC-derived early ventricular cardiac myocytes. Stem Cell Res. 2013;11:1335–47. https://doi.org/10.1016/j.scr.2013.09.003.
Block T, Creech J, da Rocha AM, Marinkovic M, Ponce-Balbuena D, Jiménez-Vázquez EN, Griffey S, Herron TJ. Human perinatal stem cell derived extracellular matrix enables rapid maturation of hiPSC-CM structural and functional phenotypes. Sci Rep. 2020;10:19071. https://doi.org/10.1038/s41598-020-76052-y.
Bowen TJ, Hall AR, Lloyd GR, Weber RJM, Wilson A, Pointon A, Viant MR. An extensive metabolomics workflow to discover cardiotoxin-induced molecular perturbations in microtissues. Metabolites. 2021;11(9):644. https://doi.org/10.3390/metabo11090644.
Bruhn J, Malmborg M, Garred CH, Ravn P, Zahir D, Andersson C, Gislason G, Torp-Pedersen C, Kragholm K, Fosbol E, et al. Temporal trends in the incidence of malignancy in heart failure: a nationwide Danish study. Eur Heart J. 2023. https://doi.org/10.1093/eurheartj/ehac797.
Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11:855–60. https://doi.org/10.1038/nmeth.2999.
Burridge PW, Li YF, Matsa E, Wu H, Ong SG, Sharma A, Holmström A, Chang AC, Coronado MJ, Ebert AD, et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med. 2016;22:547–56. https://doi.org/10.1038/nm.4087.
Campostrini G, Meraviglia V, Giacomelli E, van Helden RWJ, Yiangou L, Davis RP, Bellin M, Orlova VV, Mummery CL. Generation, functional analysis and applications of isogenic three-dimensional self-aggregating cardiac microtissues from human pluripotent stem cells. Nat Protoc. 2021;16:2213–56. https://doi.org/10.1038/s41596-021-00497-2.
Cheung YF, Li VW, Lai CT, Shin VY, Keung W, Cheuk DK, Kwong A, Li RA, Chan GC. Circulating high-sensitivity troponin T and microRNAs as markers of myocardial damage during childhood leukaemia treatment. Pediatr Res. 2021;89:1245–52. https://doi.org/10.1038/s41390-020-1049-5.
Chikae S, Kubota A, Nakamura H, Oda A, Yamanaka A, Akagi T, Akashi M. Bioprinting 3D human cardiac tissue chips using the pin type printer “microscopic painting device” and analysis for cardiotoxicity. Biomed Mater. 2021;16:025017. https://doi.org/10.1088/1748-605X/abdbde.
Chramiec A, Teles D, Yeager K, Marturano-Kruik A, Pak J, Chen T, Hao L, Wang M, Lock R, Tavakol DN, et al. Integrated human organ-on-a-chip model for predictive studies of anti-tumor drug efficacy and cardiac safety. Lab Chip. 2020;20:4357–72. https://doi.org/10.1039/d0lc00424c.
Cruz-Moreira D, Visone R, VasquesNovoa FASB, Leite-Moreira A, Redaelli A, Moretti M, Rasponi M. Assessing the influence of perfusion on cardiac microtissue maturation: a heart-on-chip platform embedding peristaltic pump capabilities. Biotechnol Bioeng. 2021;118:3128–37. https://doi.org/10.1002/bit.27836.
Cyganek L, Tiburcy M, Sekeres K, Gerstenberg K, Bohnenberger H, Lenz C, Henze S, Stauske M, Salinas G, Zimmermann WH, et al. Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI Insight. 2018;3(12):e99941. https://doi.org/10.1172/jci.insight.99941.
de Wit S, de Boer RA. From studying heart disease and cancer simultaneously to reverse cardio-oncology. Circulation. 2021;144:93–5. https://doi.org/10.1161/circulationaha.120.053315.
de Wit S, Glen C, de Boer RA, Lang NN. Mechanisms shared between cancer, heart failure, and targeted anti-cancer therapies. Cardiovasc Res. 2023;118:3451–66. https://doi.org/10.1093/cvr/cvac132.
Devalla HD, Schwach V, Ford JW, Milnes JT, El-Haou S, Jackson C, Gkatzis K, Elliott DA, de Chuva Sousa Lopes SM, Mummery CL, et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol Med. 2015;7:394–410. https://doi.org/10.15252/emmm.201404757.
Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG, Gramolini A, Keller G. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29:1011–8. https://doi.org/10.1038/nbt.2005.
Elliott DA, Braam SR, Koutsis K, Ng ES, Jenny R, Lagerqvist EL, Biben C, Hatzistavrou T, Hirst CE, Yu QC, et al. NKX2-5eGFP/w hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods. 2011;8:1037–40. https://doi.org/10.1038/nmeth.1740.
Feric NT, Pallotta I, Singh R, Bogdanowicz DR, Gustilo M, Chaudhary K, Willette RN, Chendrimada T, Xu X, Graziano MP, Aschar-Sobbi R. Engineered cardiac tissues generated in the Biowire™ II: a platform for human-based drug discovery. Toxicol Sci: Off J Soc Toxicol. 2019;172:89–97. https://doi.org/10.1093/toxsci/kfz168.
Feyen DAM, McKeithan WL, Bruyneel AAN, Spiering S, Hörmann L, Ulmer B, Zhang H, Briganti F, Schweizer M, Hegyi B, et al. Metabolic maturation media improve physiological function of human iPSC-derived cardiomyocytes. Cell Rep. 2020;32:107925. https://doi.org/10.1016/j.celrep.2020.107925.
Focaccetti C, Bruno A, Magnani E, Bartolini D, Principi E, Dallaglio K, Bucci EO, Finzi G, Sessa F, Noonan DM, Albini A. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS ONE. 2015;10:e0115686. https://doi.org/10.1371/journal.pone.0115686.
Forghani P, Rashid A, Sun F, Liu R, Li D, Lee MR, Hwang H, Maxwell JT, Mandawat A, Wu R, et al. Carfilzomib treatment causes molecular and functional alterations of human induced pluripotent stem cell-derived cardiomyocytes. J Am Heart Assoc. 2021;10:e022247. https://doi.org/10.1161/jaha.121.022247.
Fukushima H, Yoshioka M, Kawatou M, Lopez-Davila V, Takeda M, Kanda Y, Sekino Y, Yoshida Y, Yamashita JK. Specific induction and long-term maintenance of high purity ventricular cardiomyocytes from human induced pluripotent stem cells. PLoS ONE. 2020;15:e0241287. https://doi.org/10.1371/journal.pone.0241287.
Gardin C, Ferroni L, Latremouille C, Chachques JC, Mitrecic D, Zavan B. Recent applications of three dimensional printing in cardiovascular medicine. Cells. 2020;9(3):742. https://doi.org/10.3390/cells9030742.
Giacomelli E, Meraviglia V, Campostrini G, Cochrane A, Cao X, van Helden RWJ, Krotenberg Garcia A, Mircea M, Kostidis S, Davis RP, et al. Human-iPSC-derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell. 2020;26:862-879.e811. https://doi.org/10.1016/j.stem.2020.05.004.
Goldfracht I, Efraim Y, Shinnawi R, Kovalev E, Huber I, Gepstein A, Arbel G, Shaheen N, Tiburcy M, Zimmermann WH, et al. Engineered heart tissue models from hiPSC-derived cardiomyocytes and cardiac ECM for disease modeling and drug testing applications. Acta Biomater. 2019;92:145–59. https://doi.org/10.1016/j.actbio.2019.05.016.
Hattori F, Chen H, Yamashita H, Tohyama S, Satoh YS, Yuasa S, Li W, Yamakawa H, Tanaka T, Onitsuka T, et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods. 2010;7:61–6. https://doi.org/10.1038/nmeth.1403.
Holmgren G, Synnergren J, Bogestål Y, Améen C, Åkesson K, Holmgren S, Lindahl A, Sartipy P. Identification of novel biomarkers for doxorubicin-induced toxicity in human cardiomyocytes derived from pluripotent stem cells. Toxicology. 2015;328:102–11. https://doi.org/10.1016/j.tox.2014.12.018.
Ionta V, Liang W, Kim EH, Rafie R, Giacomello A, Marbán E, Cho HC. SHOX2 overexpression favors differentiation of embryonic stem cells into cardiac pacemaker cells, improving biological pacing ability. Stem Cell Reports. 2015;4:129–42. https://doi.org/10.1016/j.stemcr.2014.11.004.
Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95.
Jung JJ, Husse B, Rimmbach C, Krebs S, Stieber J, Steinhoff G, Dendorfer A, Franz WM, David R. Programming and isolation of highly pure physiologically and pharmacologically functional sinus-nodal bodies from pluripotent stem cells. Stem Cell Reports. 2014;2:592–605. https://doi.org/10.1016/j.stemcr.2014.03.006.
Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–40. https://doi.org/10.1016/j.stem.2010.12.008.
Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–14. https://doi.org/10.1172/jci12131.
Kerr CM, Richards D, Menick DR, Deleon-Pennell KY, Mei Y. Multicellular human cardiac organoids transcriptomically model distinct tissue-level features of adult myocardium. Int J Mol Sci. 2021;22(16):8482. https://doi.org/10.3390/ijms22168482.
Kim SY, Kim SJ, Kim BJ, Rah SY, Chung SM, Im MJ, Kim UH. Doxorubicin-induced reactive oxygen species generation and intracellular Ca2+ increase are reciprocally modulated in rat cardiomyocytes. Exp Mol Med. 2006;38:535–45. https://doi.org/10.1038/emm.2006.63.
Kitani T, Ong SG, Lam CK, Rhee JW, Zhang JZ, Oikonomopoulos A, Ma N, Tian L, Lee J, Telli ML, et al. Human-induced pluripotent stem cell model of trastuzumab-induced cardiac dysfunction in patients with breast cancer. Circulation. 2019;139:2451–65. https://doi.org/10.1161/circulationaha.118.037357.
Kleinsorge M, Cyganek L. Subtype-directed differentiation of human iPSCs into atrial and ventricular cardiomyocytes. STAR Protocols. 2020;1:100026. https://doi.org/10.1016/j.xpro.2020.100026.
Kofron CM, Kim TY, Munarin F, Soepriatna AH, Kant RJ, Mende U, Choi BR, Coulombe KLK. A predictive in vitro risk assessment platform for pro-arrhythmic toxicity using human 3D cardiac microtissues. Sci Rep. 2021;11:10228. https://doi.org/10.1038/s41598-021-89478-9.
Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. https://doi.org/10.1038/nbt1327.
Lau S, Rangarajan R, Philidet C, Krüger-Genge A, Braune S, Kammerer S, Küpper JH, Lendlein A, Jung F. Effects of acrolein in comparison to its prodrug cyclophosphamide on human primary endothelial cells in vitro. Toxicol in Vitro. 2020;62:104685. https://doi.org/10.1016/j.tiv.2019.104685.
Lee JH, Protze SI, Laksman Z, Backx PH, Keller GM. Human pluripotent stem cell-derived atrial and ventricular cardiomyocytes develop from distinct mesoderm populations. Cell Stem Cell. 2017;21:179-194.e174. https://doi.org/10.1016/j.stem.2017.07.003.
Lee J, Mehrotra S, Zare-Eelanjegh E, Rodrigues RO, Akbarinejad A, Ge D, Amato L, Kiaee K, Fang Y, Rosenkranz A, et al. A heart-breast cancer-on-a-chip platform for disease modeling and monitoring of cardiotoxicity induced by cancer chemotherapy. Small. 2021;17:e2004258. https://doi.org/10.1002/smll.202004258.
Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–14. https://doi.org/10.1002/1097-0142(197308)32:2%3c302::aid-cncr2820320205%3e3.0.co;2-2.
Lemoine MD, Krause T, Koivumäki JT, Prondzynski M, Schulze ML, Girdauskas E, Willems S, Hansen A, Eschenhagen T, Christ T. Human induced pluripotent stem cell-derived engineered heart tissue as a sensitive test system for QT prolongation and arrhythmic triggers. Circ Arrhythm Electrophysiol. 2018;11:e006035. https://doi.org/10.1161/circep.117.006035.
Levato R, Jungst T, Scheuring RG, Blunk T, Groll J, Malda J. From shape to function: the next step in bioprinting. Adv Mater. 2020;32:e1906423. https://doi.org/10.1002/adma.201906423.
Li Y, Tian W, Yue D, Chen C, Li C, Zhang Z, Wang C. Bevacizumab-induced mitochondrial dysfunction, endoplasmic reticulum stress, and ERK inactivation contribute to cardiotoxicity. Oxid Med Cell Longev. 2021;2021:5548130. https://doi.org/10.1155/2021/5548130.
Li Y, Zhang Y, Zhou X, Lei X, Li X, Wei L. Dynamic observation of 5-fluorouracil-induced myocardial injury and mitochondrial autophagy in aging rats. Exp Ther Med. 2021;22:1451. https://doi.org/10.3892/etm.2021.10886.
Li D, Song C, Zhang J, Zhao X. ROS and iron homeostasis dependent ferroptosis play a vital role in 5-Fluorouracil induced cardiotoxicity in vitro and in vivo. Toxicology. 2022;468:153113. https://doi.org/10.1016/j.tox.2022.153113.
Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:E1848-1857. https://doi.org/10.1073/pnas.1200250109.
Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 2012;8:162–75. https://doi.org/10.1038/nprot.2012.150.
Liu H, Bolonduro OA, Hu N, Ju J, Rao AA, Duffy BM, Huang Z, Black LD, Timko BP. Heart-on-a-chip model with integrated extra- and intracellular bioelectronics for monitoring cardiac electrophysiology under acute hypoxia. Nano Lett. 2020;20:2585–93. https://doi.org/10.1021/acs.nanolett.0c00076.
Liu C, Feng X, Li G, Gokulnath P, Xiao J. Generating 3D human cardiac constructs from pluripotent stem cells. EBioMedicine. 2022;76:103813. https://doi.org/10.1016/j.ebiom.2022.103813.
Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, Tocchetti CG, Moslehi JJ, Groarke JD, Bergler-Klein J, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22:1945–60. https://doi.org/10.1002/ejhf.1920.
Lyu YL, Kerrigan JE, Lin C-P, Azarova AM, Tsai Y-C, Ban Y, Liu LF. Topoisomerase IIβ–mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Can Res. 2007;67:8839–46. https://doi.org/10.1158/0008-5472.can-07-1649.
Ma W, Liu M, Liang F, Zhao L, Gao C, Jiang X, Zhang X, Zhan H, Hu H, Zhao Z. Cardiotoxicity of sorafenib is mediated through elevation of ROS level and CaMKII activity and dysregulation of calcium homoeostasis. Basic Clin Pharmacol Toxicol. 2020;126:166–80. https://doi.org/10.1111/bcpt.13318.
Magdy T, Jouni M, Kuo HH, Weddle CJ, Lyra-Leite D, Fonoudi H, Romero-Tejeda M, Gharib M, Javed H, Fajardo G, et al. Identification of drug transporter genomic variants and inhibitors that protect against doxorubicin-induced cardiotoxicity. Circulation. 2022;145:279–94. https://doi.org/10.1161/circulationaha.121.055801.
Mannhardt I, Eder A, Dumotier B, Prondzynski M, Krämer E, Traebert M, Söhren KD, Flenner F, Stathopoulou K, Lemoine MD, et al. Blinded contractility analysis in hiPSC-cardiomyocytes in engineered heart tissue format: comparison with human atrial trabeculae. Toxicol Sci: Off J Soc Toxicol. 2017;158:164–75. https://doi.org/10.1093/toxsci/kfx081.
Marsano A, Conficconi C, Lemme M, Occhetta P, Gaudiello E, Votta E, Cerino G, Redaelli A, Rasponi M. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip. 2016;16:599–610. https://doi.org/10.1039/c5lc01356a.
McKeithan WL, Feyen DAM, Bruyneel AAN, Okolotowicz KJ, Ryan DA, Sampson KJ, Potet F, Savchenko A, Gómez-Galeno J, Vu M, et al. Reengineering an antiarrhythmic drug using patient hiPSC cardiomyocytes to improve therapeutic potential and reduce toxicity. Cell Stem Cell. 2020;27:813-821.e816. https://doi.org/10.1016/j.stem.2020.08.003.
Michel L, Helfrich I, Hendgen-Cotta UB, Mincu RI, Korste S, Mrotzek SM, Spomer A, Odersky A, Rischpler C, Herrmann K, et al. Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur Heart J. 2022;43:316–29. https://doi.org/10.1093/eurheartj/ehab430.
Miki K, Endo K, Takahashi S, Funakoshi S, Takei I, Katayama S, Toyoda T, Kotaka M, Takaki T, Umeda M, et al. Efficient detection and purification of cell populations using synthetic MicroRNA switches. Cell Stem Cell. 2015;16:699–711. https://doi.org/10.1016/j.stem.2015.04.005.
Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, Kramer J, Siegel RL. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409–36. https://doi.org/10.3322/caac.21731.
Moslehi J, Zhang Q, Moore KJ. Crosstalk between the heart and cancer: beyond drug toxicity. Circulation. 2020;142:684–7. https://doi.org/10.1161/CIRCULATIONAHA.120.048655.
Narkar A, Willard JM, Blinova K. Chronic cardiotoxicity assays using human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs). Int J Mol Sci. 2022;23(6):3199. https://doi.org/10.3390/ijms23063199.
Nguyen N, Souza T, Verheijen MCT, Gmuender H, Selevsek N, Schlapbach R, Kleinjans J, Jennen D. Translational proteomics analysis of anthracycline-induced cardiotoxicity from cardiac microtissues to human heart biopsies. Front Gen. 2021;12:695625. https://doi.org/10.3389/fgene.2021.695625.
Nguyen N, Lienhard M, Herwig R, Kleinjans J, Jennen D. Epirubicin alters DNA methylation profiles related to cardiotoxicity. Front Biosci. 2022;27:173. https://doi.org/10.31083/j.fbl2706173.
Nguyen N, Souza T, Kleinjans J, Jennen D. Transcriptome analysis of long noncoding RNAs reveals their potential roles in anthracycline-induced cardiotoxicity. Non-Coding RNA Res. 2022;7:106–13. https://doi.org/10.1016/j.ncrna.2022.01.002.
Okwuosa TM, Barac A. Burgeoning cardio-oncology programs. J Am Coll Cardiol. 2015;66:1193–7. https://doi.org/10.1016/j.jacc.2015.07.033.
Omole JG, Ayoka OA, Alabi QK, Adefisayo MA, Asafa MA, Olubunmi BO, Fadeyi BA. Protective effect of kolaviron on cyclophosphamide-induced cardiac toxicity in rats. J Evid-Based Integr Med. 2018;23:215658721875764. https://doi.org/10.1177/2156587218757649.
Paloschi V, Sabater-Lleal M, Middelkamp H, Vivas A, Johansson S, van der Meer A, Tenje M, Maegdefessel L. Organ-on-a-chip technology: a novel approach to investigate cardiovascular diseases. Cardiovasc Res. 2021;117:2742–54. https://doi.org/10.1093/cvr/cvab088.
Pan JA, Zhang H, Lin H, Gao L, Zhang HL, Zhang JF, Wang CQ, Gu J. Irisin ameliorates doxorubicin-induced cardiac perivascular fibrosis through inhibiting endothelial-to-mesenchymal transition by regulating ROS accumulation and autophagy disorder in endothelial cells. Redox Biol. 2021;46:102120. https://doi.org/10.1016/j.redox.2021.102120.
Peng J, Dong C, Wang C, Li W, Yu H, Zhang M, Zhao Q, Zhu B, Zhang J, Li W, et al. Cardiotoxicity of 5-fluorouracil and capecitabine in Chinese patients: a prospective study. Cancer Commun. 2018;38:22. https://doi.org/10.1186/s40880-018-0292-1.
Pitaktong I, Lui C, Lowenthal J, Mattson G, Jung WH, Bai Y, Yeung E, Ong CS, Chen Y, Gerecht S, Hibino N. Early vascular cells improve microvascularization within 3D cardiac spheroids. Tissue engineering. Part C, Methods. 2020;26:80–90. https://doi.org/10.1089/ten.TEC.2019.0228.
Podgurskaya AD, Slotvitsky MM, Tsvelaya VA, Frolova SR, Romanova SG, Balashov VA, Agladze KI. Cyclophosphamide arrhythmogenicitytesting using human-induced pluripotent stem cell-derived cardiomyocytes. Sci Rep. 2021;11(1):2336. https://doi.org/10.1038/s41598-020-79085-5.
Polonchuk L, Chabria M, Badi L, Hoflack JC, Figtree G, Davies MJ, Gentile C. Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci Rep. 2017;7:7005. https://doi.org/10.1038/s41598-017-06385-8.
Protze SI, Liu J, Nussinovitch U, Ohana L, Backx PH, Gepstein L, Keller GM. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat Biotechnol. 2017;35:56–68. https://doi.org/10.1038/nbt.3745.
Richards DJ, Li Y, Kerr CM, Yao J, Beeson GC, Coyle RC, Chen X, Jia J, Damon B, Wilson R, et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat Biomed Eng. 2020;4:446–62. https://doi.org/10.1038/s41551-020-0539-4.
Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–43. https://doi.org/10.1038/s41586-018-0016-3.
Ronaldson-Bouchard K, Teles D, Yeager K, Tavakol DN, Zhao Y, Chramiec A, Tagore S, Summers M, Stylianos S, Tamargo M, et al. A multi-organ chip with matured tissue niches linked by vascular flow. Nat Biomed Eng. 2022;6:351–71. https://doi.org/10.1038/s41551-022-00882-6.
Ross JS, Fletcher JA. The HER-2/neu oncogene: prognostic factor, predictive factor and target for therapy. Semin Cancer Biol. 1999;9:125–38. https://doi.org/10.1006/scbi.1998.0083.
Sapp V, Aguirre A, Mainkar G, Ding J, Adler E, Liao R, Sharma S, Jain M. Genome-wide CRISPR/Cas9 screening in human iPS derived cardiomyocytes uncovers novel mediators of doxorubicin cardiotoxicity. Sci Rep. 2021;11:13866. https://doi.org/10.1038/s41598-021-92988-1.
Sasaki R, Kurebayashi N, Eguchi H, Horimoto Y, Shiga T, Miyazaki S, Kashiyama T, Akamatsu W, Saito M. Involvement of kallikrein-PAR2-proinflammatory pathway in severe trastuzumab-induced cardiotoxicity. Cancer Sci. 2022;113:3449–62. https://doi.org/10.1111/cas.15508.
Schneider O, Zeifang L, Fuchs S, Sailer C, Loskill P. User-friendly and parallelized generation of human induced pluripotent stem cell-derived microtissues in a centrifugal heart-on-a-chip. Tissue Eng Part A. 2019;25:786–98. https://doi.org/10.1089/ten.TEA.2019.0002.
Schwach V, Verkerk AO, Mol M, Monshouwer-Kloots JJ, Devalla HD, Orlova VV, Anastassiadis K, Mummery CL, Davis RP, Passier R. A COUP-TFII human embryonic stem cell reporter line to identify and select atrial cardiomyocytes. Stem Cell Reports. 2017;9:1765–79. https://doi.org/10.1016/j.stemcr.2017.10.024.
Shaheen N, Shiti A, Huber I, Shinnawi R, Arbel G, Gepstein A, Setter N, Goldfracht I, Gruber A, Chorna SV, Gepstein L. Human induced pluripotent stem cell-derived cardiac cell sheets expressing genetically encoded voltage indicator for pharmacological and arrhythmia studies. Stem Cell Reports. 2018;10:1879–94. https://doi.org/10.1016/j.stemcr.2018.04.006.
Sharma A, McKeithan WL, Serrano R, Kitani T, Burridge PW, Del Álamo JC, Mercola M, Wu JC. Use of human induced pluripotent stem cell-derived cardiomyocytes to assess drug cardiotoxicity. Nat Protoc. 2018;13:3018–41. https://doi.org/10.1038/s41596-018-0076-8.
Sharma A, Burridge PW, McKeithan WL, Serrano R, Shukla P, Sayed N, Churko JM, Kitani T, Wu H, Holmström A, et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Trans Med. 2017;9. https://doi.org/10.1126/scitranslmed.aaf2584.
Südhoff T, Enderle MD, Pahlke M, Petz C, Teschendorf C, Graeven U, Schmiegel W. 5-Fluorouracil induces arterial vasocontractions. Ann Oncol. 2004;15:661–4. https://doi.org/10.1093/annonc/mdh150.
Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K, Ishikita A, Matsushima S, Koumura T, Yamada K-I, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5(9):e132747. https://doi.org/10.1172/jci.insight.132747.
Tanaka Y, Nagoshi T, Yoshii A, Oi Y, Takahashi H, Kimura H, Ito K, Kashiwagi Y, Tanaka TD, Yoshimura M. Xanthine oxidase inhibition attenuates doxorubicin-induced cardiotoxicity in mice. Free Radic Biol Med. 2021;162:298–308. https://doi.org/10.1016/j.freeradbiomed.2020.10.303.
Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12:127–37. https://doi.org/10.1016/j.stem.2012.09.013.
Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis Oncol. 2018;2:13. https://doi.org/10.1038/s41698-018-0056-z.
Truitt R, Mu A, Corbin EA, Vite A, Brandimarto J, Ky B, Margulies KB. Increased afterload augments sunitinib-induced cardiotoxicity in an engineered cardiac microtissue model. JACC Basic Trans Sci. 2018;3:265–76. https://doi.org/10.1016/j.jacbts.2017.12.007.
Uosaki H, Fukushima H, Takeuchi A, Matsuoka S, Nakatsuji N, Yamanaka S, Yamashita JK. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS ONE. 2011;6:e23657. https://doi.org/10.1371/journal.pone.0023657.
Varricchi G, Galdiero MR, Marone G, Criscuolo G, Triassi M, Bonaduce D, Marone G, Tocchetti CG. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open. 2017;2:e000247. https://doi.org/10.1136/esmoopen-2017-000247.
Visone R, Lozano-Juan F, Marzorati S, Rivolta MW, Pesenti E, Redaelli A, Sassi R, Rasponi M, Occhetta P. Predicting human cardiac QT alterations and pro-arrhythmic effects of compounds with a 3D beating heart-on-chip platform. Toxicol Sci: Off J Soc Toxicol. 2022. https://doi.org/10.1093/toxsci/kfac108.
Vučković S, Dinani R, Nollet EE, Kuster DWD, Buikema JW, Houtkooper RH, Nabben M, van der Velden J, Goversen B. Characterization of cardiac metabolism in iPSC-derived cardiomyocytes: lessons from maturation and disease modeling. Stem Cell Res Ther. 2022;13:332. https://doi.org/10.1186/s13287-022-03021-9.
Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20:616–23. https://doi.org/10.1038/nm.3545.
Wang H, Wei J, Zheng Q, Meng L, Xin Y, Yin X, Jiang X. Radiation-induced heart disease: a review of classification, mechanism and prevention. Int J Biol Sci. 2019;15:2128–38. https://doi.org/10.7150/ijbs.35460.
Weng KC, Kurokawa YK, Hajek BS, Paladin JA, Shirure VS, George SC. Human induced pluripotent stem-cardiac-endothelial-tumor-on-a-chip to assess anticancer efficacy and cardiotoxicity. Tissue Eng Part C, Methods. 2020;26:44–55. https://doi.org/10.1089/ten.TEC.2019.0248.
Wiesemann A, Ketteler J, Slama A, Wirsdörfer F, Hager T, Röck K, Engel DR, Fischer JW, Aigner C, Jendrossek V, Klein D. Inhibition of radiation-induced Ccl2 signaling protects lungs from vascular dysfunction and endothelial cell loss. Antioxid Redox Signal. 2019;30:213–31. https://doi.org/10.1089/ars.2017.7458.
Winbo A, Ramanan S, Eugster E, Jovinge S, Skinner JR, Montgomery JM. Functional coculture of sympathetic neurons and cardiomyocytes derived from human-induced pluripotent stem cells. Am J Physiol Heart Circ Physiol. 2020;319:H927-h937. https://doi.org/10.1152/ajpheart.00546.2020.
Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–8. https://doi.org/10.1161/01.res.0000035254.80718.91.
Xu H, Liu G, Gong J, Zhang Y, Gu S, Wan Z, Yang P, Nie Y, Wang Y, Huang ZP, et al. Investigating and resolving cardiotoxicity induced by COVID-19 treatments using human pluripotent stem cell-derived cardiomyocytes and engineered heart tissues. Adv Sci. 2022;9:e2203388. https://doi.org/10.1002/advs.202203388.
Xu Z, Gao Z, Fu H, Zeng Y, Jin Y, Xu B, Zhang Y, Pan Z, Chen X, Zhang X, et al. PTX3 from vascular endothelial cells contributes to trastuzumab-induced cardiac complications. Cardiovasc Res. 2023;119:1250–64. https://doi.org/10.1093/cvr/cvad012.
Yadid M, Lind JU, Ardona HAM, Sheehy SP, Dickinson LE, Eweje F, Bastings MMC, Pope B, O'Connor BB, Straubhaar JR, et al. Endothelial extracellular vesicles contain protective proteins and rescue ischemia-reperfusion injury in a human heart-on-chip. Sci Trans Med 2020;12(565):eaax8005. https://doi.org/10.1126/scitranslmed.aax8005.
Yamauchi K, Li J, Morikawa K, Liu L, Shirayoshi Y, Nakatsuji N, Elliott DA, Hisatome I, Suemori H. Isolation and characterization of ventricular-like cells derived from NKX2-5(eGFP/w) and MLC2v(mCherry/w) double knock-in human pluripotent stem cells. Biochem Biophys Res Commun. 2018;495:1278–84. https://doi.org/10.1016/j.bbrc.2017.11.133.
Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–8. https://doi.org/10.1038/nature06894.
Yang L, Gong Y, Tan Y, Wu L, Witman N, Zheng J, Zhang J, Fu W, Wang W. Dexmedetomidine exhibits antiarrhythmic effects on human-induced pluripotent stem cell-derived cardiomyocytes through a Na/Ca channel-mediated mechanism. Ann Trans Med. 2021;9:399. https://doi.org/10.21037/atm-20-5898.
Yang X, Ribeiro AJS, Pang L, Strauss DG. Use of human iPSC-CMs in nonclinical regulatory studies for cardiac safety assessment. Toxicol Sci. 2022;190:117–26. https://doi.org/10.1093/toxsci/kfac095.
Yin F, Zhang X, Wang L, Wang Y, Zhu Y, Li Z, Tao T, Chen W, Yu H, Qin J. HiPSC-derived multi-organoids-on-chip system for safety assessment of antidepressant drugs. Lab Chip. 2021;21:571–81. https://doi.org/10.1039/d0lc00921k.
Zhang Q, Jiang J, Han P, Yuan Q, Zhang J, Zhang X, Xu Y, Cao H, Meng Q, Chen L, et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21:579–87. https://doi.org/10.1038/cr.2010.163.
Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell’Erba V, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. https://doi.org/10.1016/j.biomaterials.2016.09.003.
Zhang JZ, Termglinchan V, Shao NY, Itzhaki I, Liu C, Ma N, Tian L, Wang VY, Chang ACY, Guo H, et al. A human iPSC double-reporter system enables purification of cardiac lineage subpopulations with distinct function and drug response profiles. Cell Stem Cell. 2019;24:802-811.e805. https://doi.org/10.1016/j.stem.2019.02.015.
Zhang X, Hu C, Kong C-Y, Song P, Wu H-M, Xu S-C, Yuan Y-P, Deng W, Ma Z-G, Tang Q-Z. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 2019;27:540–55. https://doi.org/10.1038/s41418-019-0372-z.
Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, Aggarwal P, Zhang B, Conant G, Ronaldson-Bouchard K, et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell. 2019;176(913–927):e918. https://doi.org/10.1016/j.cell.2018.11.042.
Zhen J, Yu H, Ji H, Cai L, Leng J, Keller BB. Neonatal murine engineered cardiac tissue toxicology model: impact of dexrazoxane on doxorubicin induced injury. Life Sci. 2019;239:117070. https://doi.org/10.1016/j.lfs.2019.117070.
Zhou Q, Hu W, Fei X, Huang X, Chen X, Zhao D, Huang J, Jiang L, Wang G. Recombinant human neuregulin-1β is protective against radiation-induced myocardial cell injury. Mol Med Rep. 2016;14:325–30. https://doi.org/10.3892/mmr.2016.5207.
Funding
This work was supported by British Heart Foundation (PG/15/11/31279, PG/15/86/31723, PG/16/1/31892, PG/20/10458, and PG/23/11371) and the National Natural Sciences Foundation of China (82174196). This work forms part of the research portfolio for the National Institute for Health Research Biomedical Research Centre at Barts.
Author information
Authors and Affiliations
Contributions
S.L. and C.F. performed the literature search and made a draft, C.Z. revised and commented on the manuscript. J.L. and Q.X. generated research funds and edited the manuscript. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Fang, C., Zhong, C. et al. Recent advances in pluripotent stem cell-derived cardiac organoids and heart-on-chip applications for studying anti-cancer drug-induced cardiotoxicity. Cell Biol Toxicol 39, 2527–2549 (2023). https://doi.org/10.1007/s10565-023-09835-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-023-09835-4