Abstract
Leaf litter constitutes a major fraction in total litter production in mangrove forests. Its contribution to NPP of mangrove forests and carbon sequestration is less studied. These aspects were investigated for Kunhimangalam mangrove forest in Kerala, India. We quantified the leaf litter production and estimated the decomposition rates of leaf litter from Aegiceras corniculatum, Avicennia officinalis, Excoecaria agallocha and Rhizophora mucronata. These four species together constituted 92.49% of abundance in Kunhimangalam. The average annual leaf litter production was 8.83 ± 0.95 t ha−1yr−1, 78% of the total litter produced. Leaf litter production was negatively correlated with soil pH (R2 = 0.531) and rainfall (R2 = 0.561). Temperature, salinity and humidity did not show any remarkable influence. The rate of decomposition varied significantly among these species (F = 2497.79, p < 0.01) but as for the mixed leaf litter category (a mixture of leaves from the above four species in equal weight), the rate of decomposition was the highest. The pattern of leaf litter decomposition observed in mixed, R. mucronata and E. agallocha categories best fitted to the exponential decay model indicating an initial phase of faster decomposition followed by a terminal slow phase. The leaf litter of A. corniculatum and A. officinalis categories fitted best to the linear regression model showing a steady pace throughout the period of decomposition. Leaf litter of these four species together contributed 3.56 ± 0.01 t C ha−1 y−1 to NPP. Higher production of leaf litter in Kunhimangalam showed higher potential for carbon sequestration. However, only less than 1% (0.62%) of the leaf litter was decomposed when macrobenthos were excluded from the system. The destiny of the rest 99% appeared critical as this determined the capacity of Kunhimangalam mangrove forest to act as a source or sink for carbon.





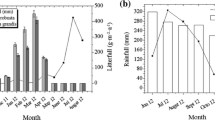
Source: Shanij et al. 2016

Source: Shanij et al. 2016







Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
NA.
References
Ake-Castilho JA, Vazques G, Lopez-Portilho J (2006) Litterfall and decomposition of Rhizophora mangle L. in a coastal lagoon in the southern Gulf of Mexico. Hydrobiologia 559:101–111
Alongi DM (2009) Paradigm shifts in mangrove biology. In: Perillo GME, Wolanski E, Cahoon DR, Brinson MM (eds) Coastal wetlands: an integrated ecosystem approach. Elsevier Publishing, Amsterdam, pp 615–640
Alongi DM (2014) Carbon cycling and storage in mangrove forests. Annu Rev Mar Sci 6(1):195–219
Alongi DM, Boto KG, Robertson AI (1992) Nitrogen and phosphorus cycles. In: Robertson AI, Alongi DM (eds) Tropical Mangrove ecosystems. American Geophysical Union, Washington DC, pp 251–292
Alongi DM, Ayukai T, Brunskill GJ, Clough BF, Wolanski E (1998) Sources, sinks, and export of organic carbon through a tropical, semi-enclosed delta (Hinchinbrook Channel, Australia. Mangroves and Salt Marshes 2:237–242
Alongi DM, Clough BF, Robertson AI (2005) Nutrient-use efficiency in arid-zone forests of the mangroves Rhozophora stylosa and Avicennia marina. Aquat Bot 82(2):121–131
Alvim EACC, Medeiros AO, Rezende RS, Goncalves JF Jr (2015) Leaf breakdown in a natural open tropical stream. J Limnol 74(2):248–260. https://doi.org/10.4081/jlimnol.2014.982
Anderson JM, Swift MJ (1983) Decomposition in Tropical Forest. In: Sulton SL, Chadwick AC, Whitemore TC (eds) The tropical rain forest. Ecology and Management Blackwell, Oxford, pp 289–309
Ardon NM, Pringle CM (2008) Do secondary compounds inhibit microbial- and insect-mediated leaf breakdown in a tropical rainforest stream. Costa Rica? Oecologia 155:311–323
Arreola-Lizarraga JS, Flores-Verdugo FJ, Ortego-Rubio A (2004) Structure and litterfall of an arid mangrove stand on the Gulf of California, Mexico. Aquat Bo 79:137–143
Ashton EC, Hogarth PJ, Ormond R (1999) Breakdown of mangrove leaf litter in a managed mangrove forest in. Peninsular Malaysia Hydrobiologia 413:77–88
Bernini E, Rezende CE (2010) Estrutura Da vegetação em florestas de mangue do estuário do rio Paraíba do sul, Estado do Rio De Janeiro, Brasil. Acta Bot Bras 18:491–502
Betoulle JL, Fromard F, Fabre A, Puig H (2001) Characterisation of litter and its contributions to soil nutriment in a mangrove of French Guiana. Can J Bot 79:238–249
Bosire JO, Dahdouh-Guebas F, Kairo JG, Kazungu J, Dehairs F, Koedam N (2005) Litter degradation and CN dynamics in reforested mangrove plantations at Gazi Bay, Kenya. Biol Conserv 126:287–295
Boulton AJ, Boon PI (1991) A review of methodology used to measure leaf litter decomposition in lotic environments: time to turnover a new leaf? Aust J Mar Freshwater Res 42:1–43
Briones MJI, Ineson P (1996) Decomposition of eucalyptus leaves in litter mixtures. Soil Biol Biochem 28(10–11):1381–1388
Brown SM (1984) Mangrove Litter Production and Dynamics in Snedaker, C.S and Snedaker, G.J. 1984. The Mangrove Ecosystem: Research Methods. On Behalf of The Unesco/SCOR, Working Group 60 on Mangrove Ecology. Page 200–208
Chapin FS, Matson PA, Mooney HA (2002) Principles of terrestrial ecosystem ecology. Springer, New York
Cintron G, Schaeffer-Novelli Y (1983) Introduccio´n a la ecologı´a del manglar. Oficina Regional de Cienca y Tecnologı´a de la UNESCO para Ame´rica Latina y el Caribe ROSTLAC, Montevideo, Uruguay
Clough BF (1992) Primary productivity and growth of mangrove forests. In: Robertson AI, Alongi DM (eds) Tropical Mangrove ecosystems-Coastal and Estuarine Series 41. American Geophysical Union, Washington, pp 225–249
Coen LD (1988) Herbivory by crabs and the control of algal epibionts on Caribbean host corals. Oecologia 75(2):198–203
Coupland GT, Paling EI, McGuinness KA (2005) Vegetative and reproductive phenologies of four mangrove species from northern Australia. Aust J Bot 53:109–117
Cox EF, Allen JA (1999) Stand structure and productivity of the introduced Rhizophora mangle in Hawaii. Estuaries 22:276–284
Cundell AM, Brown MS, Stanford R, Mitchell R (1979) Microbial degradation of Rhizophora mangle leaves immersed in the sea. Estuar Coast Marine Sci. https://doi.org/10.1016/0302-3524(79)90041-0
Day JW Jr, Coronado-Molina C, Vera-Herrera FR, Twilley R, Rivera-Monroy VH, Alvarez-Guillen H, Day R, Conner W (1996) A seven year record of above-ground net primary production in a southeastern Mexican mangrove forest. Aquat Bot 55:39–60
Duarte CM, Cebrian J (1996) The fate of marine autotrophic production. Limnol Oceanogr 41:1758–1766
Duke NC (1999) Phenological trends with latitude in the mangrove tree Avicennia marina. J Ecol 78:113–133
Edu EAB, Nsirim LEW, Martins OO (2014) Monitoring and assessment of leaf litter dynamics in a mixed mangal forest of the Cross River Estuary, Nigeria. Int J Environ Monit Anal 2(3):163–174
Feller IC, Whigham DF, O’Neill JP, McKee KM (1999) Effects of nutrient enrichment on within-stand nutrient cycling in mangrove ecosystems in Belize. Ecology 80:2193–2205
Fernando SMC, Bandeira SO (2009) Litterfall and decomposition of mangrove species Avicennia marina and Rhizophora mucronata in Maputo Bay, Mozambique, Western Indian Ocean. J Mar Sci 8(2):173–182
Frick CM, Farrell RE, Germida JJ (1999) Assessment of phytoremediation as an in situ technique for cleaning oil-contaminated sites. PTAC Petroleum Technology Alliance, Canada, Calgary
Fyles JW, Fyles IH (1993) Interaction of Douglas-fir with red alder and salal foliage litter during decomposition. Can J for Res 23:358–361
Ghosh R, Banerjee K (2013) Inter-relationship between physico-chemical variables and litter production in mangroves of Indian Sundarbans. J Mar Sci Res Dev S 11:001. https://doi.org/10.4172/2155-9910.S11-001
Harmon ME, Nadelhoffer KJ, Blair JM (1999) Measuring decomposition, nutrient turnover, and stores in plant litter. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P (eds) Standard soil methods for long term ecological research. Oxford University Press, New York, pp 202–240
Harrison PJ, Snedaker SC, Ahmed SI, Azam F (1994) Primary producers of the arid climate mangrove ecosystem of the Indus River Delta, Pakistan: an overview. Trop Ecol 35(2):155–184
Heal OW, Anderson JM, Swift MJ (1997) Plant litter quality and decomposition: an historical overview. CAB International, Oxon
Hossain M, Hoque AKF (2008) Litter production and decomposition in mangroves - a review. Indian J for 31(2):227–238
Ibrahima A, Biyanzi P, Halima M (2008) Changes in organic compounds during leaf litter leaching: laboratory experiment on eight plant species of the Sudano-guinea of Ngaoundere. Cameroon for 1:27–33
Ibrahima A, Gillon D, Joffre R (2010) Leaf litter decomposition of Mediterranean tree species in relation to temperature and initial water imbibitions under microcosm experiment. Res J Agric Biol Sci 6:32–39
Imgraben S, Dittmann S (2008) Leaf litter dynamics and litter consumption in two temperate south Australian mangrove forest. J Sea Res 59:83–93
India State of Forest Report (2021) Forest Survey of India, Ministry of Environment Forest and Climate Change, Government of India, New Delhi, India
Jackson ML (1973) Soil Chemical Analysis. Constable and Company Ltd. Prentice Hall of India Pvt. Ltd., New Delhi, pp 10–114
Kalio ANJ (1992) A pilot study of mangrove litter production in the Bonny Estuary of Southern Nigeria. Discov Innov 4(3):71–78
Kamruzzaman Md, Basak K, Paul SK, Ahmed S, Osawa A (2019) Litterfall production, decomposition and nutrient accumulation in Sundarbans mangrove forests, Bangladesh. For Sci Technol 15(1):24–32. https://doi.org/10.1080/21580103.2018.1557566
Kathiresan K, Bingham BL (2001) Biology of mangrove and mangrove ecosystems. Adv Mar Biol 40:81–251
Kavvadias VA, Alifragis DA, Tsiontsis A, Brofas G, Stamatelos G (2001) Litterfall, litter accumulation and litter decomposition rates in four forest ecosystems in northern Greece. For Ecol Manage 144:113–127
Komiyama A, Ong JE, Poungparn S (2008) Allometry, biomass, and productivity of mangrove forests: a review. Aquat Bot 89:128–137
Lacerda LD, Conde JE, Kjerfve B, Alvarez-Leon R, Polanı´a J (2001) American mangroves. In: En Lacerda LD (ed) Mangrove ecosystems: function and management. Springer, Berlin, pp 1–62
Lee SY (1997) Potential trophic importance of the faecal material of the mangrove crab Sesarma messa. Mar Ecol Prog Ser 159:275–284
Lopez-Portilho J, Ezcurra E (1985) Litterfall of Avicennia germinans L. in a one-year cycle in a mudflat at the Laguna De Mecoacan. Tabasco Mexico Biotropica 17:186–190
Lorıa-Naranjo M, Sibaja-Cordero JA, Cortes J (2019) Mangrove leaf litter decomposition in a seasonal tropical environment. J Coast Res 35(1):122–129. https://doi.org/10.2112/JCOASTRES-D-17-00095.1
Mackey AP, Smail G (1996) The decomposition of mangrove litter in a subtropical mangrove forest. Hydrobiologia 332:93–98
Mahmood H, Saberi O, Misri K, Japar Sidik B (2007) Nutrients dynamics associated with leaf litter degradation of Bruguieria parviflora (Whight and Arnold) at Kuala Selangor Mangrove Forest, Malaysia. Indian J for 30:325–330
Mahmood H, Limon SH, Rahman MS, Azad AK, Islam MS, Khairuzzaman M (2009) Nutrients (N, P and K) dynamics associated with the leaf litter of two agro forestry tree species of Bangladesh. I for 2:183–186
Mahmood H, Siddique MRH, Rahman MS, Hossain MZ, Hasan MM (2011) Nutrient dynamics associated with leaf litter decomposition of three agro forestry tree species (Azadirachta indica, Dalbergia sissoo and Melia azadirachta) of Bangladesh. J for Res 22:577–582
Mall LP, Singh VP, Garge A (1991) Study of biomass, litter fall, litter decomposition and soil respiration in monogeneric mangrove and mixed mangrove forests of Andaman Islands. Trop Ecol 32:144–152
Mason FC (1977) Decomposition, the Institute of Biology’s studies in Biology. Edward Arnold Ltd., London, UK
May JD (1999) Spatial variation in litter production by the mangrove Avicennia marina var. Australasica in Rangaunu Harbour, New Zealand. New Zeal J Mar Fresh 33:163–172
McArthur JV, Aho JM, Rader RB, Mills GL (1994) Interspecific leaf interactions during decomposition in aquatic and floodplain ecosystems. J N Amer Benthol Soc 13:57–67
Mchenga I, Ali AI (2017) Mangrove litter production and seasonality of dominant species in Zanzibar, Tanzania. J East Afr Nat Hist 106(1):5–18
McTiernan KB, Ineson P, Coward PA (1997) Respiration and nutrient release from tree leaf litter mixtures. Oikos 78(3):527–538
Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecology 59:465–472
Mfilinge PL, Meziane T, Bachok Z, Tsuchiya M (2005a) Litter dynamic and particulate organic matter out welling from a subtropical mangrove in Okinawa Island, South Japan. Estuar Coast Shelf Sci 63:301–313
Mfilinge PL, Meziane T, Bachok Z, Tsuchiya M (2005b) Total lipid and fatty acid classes in decomposing mangrove leaves of Bruguiera gymnorhiza and Kandelia Candel: significance with respect to lipid input. J Oceanogr 61:613–622
Middleton BA, McKee KL (2001) Degradation of mangrove tissues and implications for peat formation in Belizean island forests. J Ecol 89:818–828
Morrisey D, Beard C, Morrison M, Craggs R, Lowe M (2007) The New Zealand mangrove: review of the current state of knowledge. Auckland Regional Council Technical Publication No. 325. Auckland, New Zealand
Ngoran A, Zakra N, Ballo K, Kouamé C, Zapata F, Hofman G, Van CO (2006) Litter decomposition of Acacia auriculiformis Cunn. Ex Benth. And Acacia mangium Willd. Under coconut trees on quaternary sandy soils in Ivory Coast. Biol Fertil Soils 43:102–106
Nicholson C (2009) Mangroves and crabs as ecosystem engineers in Zanzibar. Independent Study Project (ISP) Collection Paper 760. (http://digitalcollections.sit.edu/isp_collection/760, Accessed on 22/11/2022
Ochieng CA, Erftemeijer PLA (2002) Phenology, litterfall and nutrient resorption in Avicennia marina (Forssk.) Vierh in Gazi Bay, Kenya. Trees. https://doi.org/10.1007/s00468-001-0146-2
Odum EP (1980) The status of three ecosystem level hypotheses regarding salt marshes: tidal subsidy, outwelling and the detritus based food chain. In: Kennedy VS (ed) Estuarine perspectives. Academic Press, New York, pp 485–496
Odum WE, Heald EJ (1975) The detritus-based food web of an estuarine mangrove community. In: Cronin LE (ed) Estuarine Research. Academic Press, New York, pp 265–286
Park S, Kang-Hyun C (2003) Nutrient leaching from leaf litter of emergent macrophyte (Zizania latifolia) and the effects of water temperature on the leaching process. Korean J Biol Sci 7:289–294
Polunin NVC (1982) Processes contributing to the decay of reed (Phragmites australis) litter in fresh water. Arch Hydrobiol 94:182–209
Pool DJ, Lugo AE, Snedaker SC (1975) Litter production in mangrove forests of southern Florida and Puerto Rico. In: Walsh G, Snedaker S, Teas H (eds) Proceedings of the International Symposium on the Biology and Management of Mangroves. University of Florida, Gainesville, Florida, pp 213–237
Pool DJ, Lugo A, Snedaker SC (1986) Litter production in mangrove forests of Southern Florida and Puerto Rico. Reprint 14462 UPR-RUM, 213–237
Praveen VP (2014) The role of brachyuran crabs in structure, composition and recruitment of mangrove forests in Kerala, PhD Thesis, University of Kerala, Thiruvananthapuram, India
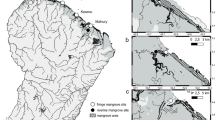
Praveen VP, Shanij K, Suresh S, Balakrishnan P (2015) Kunhimangalam, the largest mangrove in Kerala needs immediate conservation attention. SACON ENVIS Newsletter - Sarovar Saurabh 11(2):1–2
Qasim SZ, Waffar MVW (1990) Marine resource in the tropic. Resource Manage Optim 7:141–169
Ragavan P, Saxena A, Jayaraj RSC, Moha PM, Ravichandran K, Saravanan S, Vijayaraghavan A (2016) A review of the mangrove floristics of India. Taiwania 61(3):224–242. https://doi.org/10.6165/tai.2016.61.224
Rani V, Sreelekshmi S, Preethy CM, Bijoy Nandan S (2016) Phenology and litterfall dynamics structuring ecosystem productivity in a tropical mangrove stand on South West coast of India. Reg Stud Mar Sci 8:400–407. https://doi.org/10.1016/j.rsma.2016.02.008
Rani V, Sreelakshmi C, Bijoy Nandan S, Santu KS, Preethy CM (2023) Feeding ecology of Parasesarma plicatum and its relation to carbon structuring in mangrove ecosystem. Hydrobiologia 850 (4):911–927 Robertson AI, Alongi DM, Boto KG (1992) Food chains and carbon fluxes. In: Robertson AI, Alongi DM (eds) Tropical Mangrove Ecosystems. American Geophysical Union, Washington DC, pp 293–326
Saberi O (1989) The rate of litter production in mangrove forest at Siar beach. Lundu Sarawak Pertanika 12(1):47–51
Saenger P, Snedaker SC (1993) Pantropical trends in mangrove above ground biomass and annual litterfall. Oecologia 96:293–299
Salinas N, Malhi Y, Meir P, Silman M, Roman Cuesta R, Huaman J, Salinas D, Huaman V, Gibaja A, Mamani M, Farfan F (2011) The sensitivity of tropical leaf litter decomposition to temperature: results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytol 189:967–977
Shanij K (2017) Sesarmid crabs and nutrient cycling in a selected mangrove ecosystem of Kerala. Ph. D. Thesis, University of Kerala, Thiruvananthapuram, Kerala, India
Shanij K, Praveen VP, Suresh S, Oommen MM, Nayar TS (2016) Leaf litter translocation and consumption in mangrove ecosystems: the key role played by the sesarmid crab neosarmatium malabaricum. Curr Sci 110(10):1969–1976
Sherman PM (2002) Effects of land crabs on seedling densities and distributions in a mainland neotropical rain forest. J Trop Ecol 18(1):67–89
Sherman RE, Fahey TJ, Martinez P (2003) Spatial patterns of biomass and above- ground net primary productivity in a mangrove ecosystem in the Dominican Republic. Ecosystems 6:384–398
Silva CAR, Mozeto AA, Ovalle ARC (1998) Distribution and fluxes as macrodetritus of phosphorus in red mangroves, Sepetiba Bay, Brazil. Mangroves and Salt Marshes 2(1):37–42
Silva R, Silva AP, Oliveira SR (2006) Concentration, stock and transport rate of heavy metals in a tropical red mangrove, Natal, Brazil. Mar Chem 99:2–11
Simlai A, Roy A (2012) Analysis of and correlation between phytochemical and antimicrobial constituents of Ceriops Decandra, a medicinal mangrove plant, from Indian Sundarban estuary. J Med Plants Res 6:4755–4765
Slim FJ, Gwada PM, Kodjo M, Hemminga MA (1996) Biomass and litterfall of Ceriops tagal and Rhizophora mucronata in the mangrove forest of Gazi bay, Kenya. Mar Freshw Res 73:25–38
Snedaker SC, Snedaker JG (eds) (1984) The mangrove ecosystem: research methods. Monographs on Oceanographic Methodology, UNESCO, United Kingdom
Srivastava PBL (1980) Research proposals for mangrove vegetation in Malaysia. In: Srivastava PBL, Kadir RA (eds) Proceedings of Workshop on Mangrove and Estuarine Vegetation, Forest Department, Kuala Lumpur, pp 64–75
Steinke TD, Holland AJ, Singh Y (1993) Leaching losses during decomposition of mangrove leaf litter. S Afr J Bot 59:21–25
Suresh HS, Bhat DM, Ravindranath NH, Sukumar R (2017) Carbon stocks and sequestration potential of. Indian Mangroves Trop Ecol 58(3):547–553
Teal JM (1962) Energy-flow in salt-marsh ecosystem of Georgia. Ecology 43:614–624
Triadiati S, Tjitrosemito E, Sundarsono G, Qayim I, Leuschner C (2011) Litterfall production and leaf-litter decomposition at natural forest and Cacao agro forestry in Central Sulawesi, Indonesia. Asian J Biol Sci 4:221–234
Twilley RR (1995) Properties of mangrove ecosystems related to the energy signature of coastal environments. In: Hall C (ed) Maximum Power. University of Colorado Press, Boulder, Colorado, pp 43–62
Twilley RR, Chen RH, Hargis T (1992) Carbon sinks in mangroves and their implications to carbon budget of tropical coastal ecosystems. Water Air Soil Pollut 64:265–288
Twilley RR, Pozo M, Garcia VH, Rivera-Monroy VH, Zambrano R, Bodero A (1997) Litter dynamics in riverine mangrove forests in the Guayas River Estuary. Ecuador Oecologia 111:109–122
Van der Valk AG, Attiwill PM (1984) Decomposition of leaf and root litter of Avicennia marina at Westernport bay, Victoria, Australia. Aquat Bot 18:205–221
Wafar S, Untawale AG, Wafar M (1997) Litterfall and energy flux in a mangrove ecosystem. Estuar Coast Shelf Sci 44:111–124
Wangondu VW, Bosire JO, Kairo JG, Koedam N (2014) Litter fall dynamics of restored mangroves (Rhizophora mucronata Lam. And Sonneratia alba sm.) In Kenya. Restor Ecol 22(6):824–831
Wardle DA, Nilsson MC, Zackrisson O, Gallet C (2003) Determinants of litter mixing effects in a Swedish boreal forest. Soil Biol Biochem 35(6):827–835
Zaldivar Jimenez A, Herrera Silveira J, Coronado Molina C, Alonzo Parra D (2004) Estructuray Productividad De Los manglares en la reserva de biosfera Ría Celestún, Yucatán, México. Maderay Bosques Special Issue 10(1):25–35
Acknowledgements
We thank the Ministry of Environment, Forest and Climate Change, Government of India, New Delhi for financial support(D O No. 22/16/2004-CS (M) dated 09/06/2005). Dr. P. P. Moosa, Scientist in charge, Krishi Vigyan Kendra, Kerala Agricultural University, Thaliparamba, Kerala is gratefully acknowledged for providing us with data on humidity and rainfall of the study area.
Funding
The research work was funded by the Ministry of Environment, Forest and Climate Change, Government of India, New Delhi (D O No. 22/16/2004-CS (M) dated 09/06/2005).
Author information
Authors and Affiliations
Contributions
KS, SS and TSN conceptualized and designed the study and collected data in the field. KS and VJ analyzed the data. KS and SS prepared the first draft. TSN revised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Permission
The data and samples for the study were collected from an area under the authority of Payyannur Municipality and Kunhimangalam Gramapanchayat and the study was conducted with the knowledge and permission of the stakeholders.
Ethical approval
NA.
Consent to participate
NA.
Consent for publication
NA.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shanij, K., Suresh, S., Jilesh, V. et al. Leaf litter production and decomposition in a Riverine Mangrove forest in India. Wetlands Ecol Manage 32, 59–77 (2024). https://doi.org/10.1007/s11273-023-09961-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-023-09961-0