Abstract
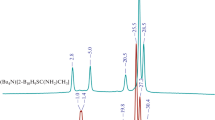
The possibility of using salts of 2,4,6-triamine-1,3,5-triazine, 2,4-diamine-6-methyl-1,3,5-triazine, and 2,4-diamine-6-phenyl-1,3,5-triazine for isolation of the dodecahydro-closo-dodecaborate anion [B12H12]2– from aqueous solutions has been studied. Compound [2,4-(NH2)2-6-Ph-1,3,5-N3С3H]2[B12H12]⋅H2O (solubility 0.06 g in 100 g water at 17°C), promising for the precipitation of the [B12H12]2– anion, has been isolated by the metathesis reaction of moderately soluble hydrochloride [2,4-(NH2)2-6-Ph-1,3,5-N3С3H]Cl⋅H2O with sodium and potassium dodecahydro-closo-dodecaborates. A procedure has been developed for the decomposition of [2,4-(NH2)2-6-Ph-1,3,5-N3С3H]2[B12H12]⋅H2O with ammonium hydroxide to obtain soluble salts of the [B12H12]2– anion.




Similar content being viewed by others
REFERENCES
B. M. Mikhailov, Chemistry of Borohydrides (Nauka, Moscow, 1967).
E. L. Muetterties, Boron Hydride Chemistry (Academic Press, New York, 1975).
I. B. Sivaev, V. I. Bregadze, and S. Sjöberg, Collect. Czech. Chem. Commun. 67, 679 (2002).
N. T. Kuznetsov, S. P. Ionov, and K. A. Solntsev, Development of the Concept of Aromaticity. Polyhedral Structures (Nauka, Moscow, 2009) [in Russian].
N. T. Kuznetsov, K. A. Solntsev, and A. V. Agafonov, Koord. Khim. 5, 1297 (1979).
A. Yu. Bykov, N. N. Mal’tseva, N. B. Generalova, et al., Russ. J. Inorg. Chem. 58, 1321 (2013). https://doi.org/10.1134/S003602361311003X
V. I. Saldin, V. V. Sukhovei, L. N. Ignat’eva, et al., Khim. Tekhnol. 20, 615 (2019). https://doi.org/10.31044/1684-5811-2019-20-13-615-619
V. Geis, K. Guttsche, C. Knapp, et al., Dalton Trans. 2687 (2009). https://doi.org/10.1039/b821030f
M. F. Hawthorne, Angew. Chem., Int. Ed. Engl. 32, 950 (1993). https://doi.org/10.1002/anie.199309501
I. B. Sivaev, V. I. Bregadze, and N. T. Kuznetsov, Russ. Chem. Bull. 51, 1362 (2002).
R. F. Barth, P. Mi, and W. Yang, Cancer Commun. 38, 35 (2018). https://doi.org/10.1186/s40880-018-0299-7
F. Ali, N. Hosmane, and Y. Zhu, Molecules 25, 828 (2020). https://doi.org/10.3390/molecules25040828
A. V. Nelyubin, N. A. Selivanov, A. Y. Bykov, et al., Int. J. Mol. Sci. 22, 13391 (2021). https://doi.org/10.3390/ijms222413391
V. V. Avdeeva, T. M. Garaev, E. A. Malinina, et al., Russ. J. Inorg. Chem. 67, 28 (2022). https://doi.org/10.1134/S0036023622010028
J. W. Johnson and J. F. Broady, J. Electrochem. Soc. 129, 2213 (1982).
L. He, H.-W. Li, H. Nakajima, et al., Chem. Mater. 27, 5483 (2015).
H. Hagemann, Molecules 26, 7425 (2021). https://doi.org/10.3390/molecules26247425
R. Bernard, D. Cornu, B. Grüner, et al., J. Organomet. Chem. 657, 83 (2002). https://doi.org/10.1016/S0022-328X(02)01540-1
T. B. Yisgedu, X. Chen, S. Schricker, et al., Chem.—Eur. J. 15, 2190 (2009). https://doi.org/10.1002/chem.200801430
I. B. Sivaev, Chem. Heterocycl. Comp. 53, 638 (2017).
Z. Zhang, Y. Zhang, Z. Li, et al., Eur. J. Inorg. Chem. 2018, 981 (2018). https://doi.org/10.1002/ejic.201701206
P. Sharon, M. Afri, S. Mitlin, et al., Polyhedron 157, 71 (2019). https://doi.org/10.1016/j.poly.2018.09.055
S. E. Korolenko, V. V. Avdeeva, E. A. Malinina, et al., Russ. J. Inorg. Chem. 66, 1350 (2021). https://doi.org/10.1134/S0036023621090047
I. B. Sivaev, Russ. J. Inorg. Chem. 66, 1289 (2021). https://doi.org/10.1134/S0036023621090151
E. A. Malinina, S. E. Korolenko, A. S. Kubasov, et al., Polyhedron 184, 114566 (2020). https://doi.org/10.1016/j.poly.2020.114566
E. Yu. Matveev, V. V. Avdeeva, K. Yu. Zhizhin, et al., Inorganics 10, 238 (2022). https://doi.org/10.3390/inorganics10120238
V. V. Avdeeva, E. A. Malinina, and N. T. Kuznetsov, Coord. Chem. Rev. 469, 214636 (2022). https://doi.org/10.1016/jccr.2022.214636
Boron Science: New Technologies and Applications, Ed. by N. S. Hosmane (CRC Press, Boca Raton, 2012). https://doi.org/10.1201/b11199
E. A. Malinina, I. I. Myshletsov, G. A. Buzanov, et al., Molecules 28, 453 (2023). https://doi.org/10.3390/molecules28010453
N. T. Kuznetsov, K. A. Solntsev, and L. N. Kulikova, Koord. Khim. 2, 1574 (1976).
V. K. Skachkova, L. V. Goeva, A. V. Grachev, et al., Russ. J. Inorg. Chem. 62, 84 (2017). https://doi.org/10.1134/S0036023617010211
V. I. Saldin, V. V. Sukhovei, L. N. Ignat’eva, et al., Theor. Found. Chem. Eng. 44, 467 (2010).
B. Bann and S. A. Miller, Chem. Rev. 58, 131 (1958). https://doi.org/10.1021/cr50019a004
Chemical Encyclopedia. Copper—Polymers, Ed. by I. L. Knunyants, vol. 3 (Bol’shaya Ross. Entsikl., Moscow, 1992) [in Russian].
V. I. Saldin, A. B. Slobodyuk, and V. V. Sukhovei, Russ. J. Inorg. Chem. 67, 1012 (2022). https://doi.org/10.1134/S003602362207021X
N. T. Kuznetsov, L. N. Kulikova, Zhurn. Anal. Khim. 31, 1312 (1976).
U. Athikomrattanakul, C. Promptmas, M. Katterle, et al., Acta Crystallogr., Sect. E 63 (2007). https://doi.org/10.1107/S1600536807014791
V. I. Saldin, L. N. Ignat’eva, V. A. Mashchenko, et al., Vestnik DVO RAN 6, 77 (2022). https://doi.org/10.37102/0869-7698_2022_226_06_7
Sh. Sheshmani, M. Ghadermazi, H. Aghabozorg, et al., Acta Crystallogr., Sect E 62 (2006). https://doi.org/10.1107/S1600536806038475
V. I. Saldin, V. V. Sukhovei, RF Patent 2617778, Byull. Izobret. 2017, No. 12.
T. Tashiro, J. Heterocycl. Chem. 39, 615 (2002). https://doi.org/10.1002/jhet.5570390402
T. Peymann, C. B. Knobler, and M. F. Hawthorne, Inorg. Chem. 39, 1163 (2000).
G. B. Seifer, Russ. J. Coord. Chem. 28, 301 (2002).
W. J. Orwill Thomas, Trans. Faraday Soc. 55, 203 (1959).
A. I. Konovalov, I. S. Ryzhkina, L. I. Murtazina, et al., Izv. Akad. Nauk., Ser. Khim. 6, 1207 (2008).
V. I. Saldin, A. B. Slobodyuk, N. N. Savchenko, et al., Russ. J. Phys. Chem. A 92, 2210 (2018). https://doi.org/10.1134/S0036024418110353
M. K. Marchewka, Mater. Sci. Eng. B 95, 214 (2002).
T. S. Miller, A. B. Jorge, T. M. Suter, et al., Phys. Chem. Chem. Phys. 19, 15613 (2017). https://doi.org/10.1039/C7CP0211G
S. V. Ivanov, E. A. Malinina, K. A. Solntsev, et al., Koord. Khim. 18, 394 (1992).
Funding
The work was carried out within the framework of the State Assignment of the Institute of Chemistry, Far Eastern Branch of the Russian Academy of Sciences (topic FWFN (205)-2022-0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Avdeeva
Rights and permissions
About this article
Cite this article
Saldin, V.I., Ignat’eva, L.N., Mashchenko, V.A. et al. Hydrochlorides and Dodecahydro-closo-dodecaborates of Amino Derivatives of 1,3,5-Triazine in the Technology of Isolation and Purification of the [B12H12]2– Anion. Russ. J. Inorg. Chem. 68, 1363–1370 (2023). https://doi.org/10.1134/S0036023623601915
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023623601915