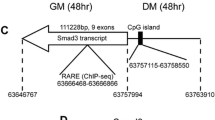
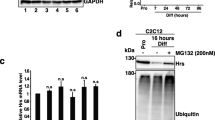
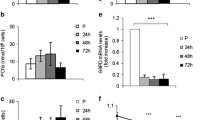
Abstract
ALDH1A1 and ALDH1A3 paralogues of aldehyde dehydrogenase 1 (ALDH1) control myogenic differentiation of skeletal muscle satellite cells (SC) by formation of retinoic acid (RA) and subsequent cell cycle adjustments. The respective relevance of each paralogue for myogenic differentiation and the mechanistic interaction of each paralogue within RA-dependent and RA-independent pathways remain elusive.
We analysed the impact of ALDH1A1 and ALDH1A3 activity on myogenesis of murine C2C12 myoblasts. Both paralogues are pivotal factors in myogenic differentiation, since CRISPR/Cas9-edited single paralogue knock-out impaired serum withdrawal-induced myogenic differentiation, while successive recombinant re-expression of ALDH1A1 or ALDH1A3, respectively, in the corresponding ALDH1 paralogue single knock-out cell lines, recovered the differentiation potential. Loss of differentiation in single knock-out cell lines was restored by treatment with RA-analogue TTNPB, while RA-receptor antagonization by AGN 193109 inhibited differentiation of wildtype cell lines, supporting the idea that RA-dependent pathway is pivotal for myogenic differentiation which is accomplished by both paralogues.
However, overexpression of ALDH1-paralogues or disulfiram-mediated inhibition of ALDH1 enzymatic activity not only increased ALDH1A1 and ALDH1A3 protein levels but also induced subsequent differentiation of C2C12 myoblasts independently from serum withdrawal, indicating that ALDH1-dependent myogenic differentiation relies on different cellular conditions. Remarkably, ALDH1-paralogue knock-out impaired the autophagic flux, namely autophagosome cargo protein p62 formation and LC3B-I to LC3B-II conversion, demonstrating that ALDH1-paralogues interact with autophagy in myogenesis. Together, ALDH1 paralogues play a crucial role in myogenesis by orchestration of complex RA-dependent and RA-independent pathways.






Similar content being viewed by others
Availability of data and material
The data and material will not be shared since all items can be purchased and therefore reproduced.
Abbreviations
- AGN:
-
AGN 193109, retinoic acid receptor antagonist
- ALDH:
-
Aldehyde dehydrogenase
- Ctrl:
-
Control
- Diff:
-
Differentiation
- DSF:
-
Disulfiram
- FACS:
-
Fluorescence-activated cell sorting
- GFP:
-
Green fluorescent protein
- KO:
-
Knock-out
- LC3B:
-
Microtubule-associated protein 1 light chain 3 beta
- MHC:
-
Myosin heavy chain
- MyoG:
-
Myogenin
- Neo:
-
Neomycin
- P62/SQSTM-1:
-
Sequestosome-1, ubiquitin-binding protein p62
- RA:
-
Retinoic acid
- RAR:
-
Retinoic acid receptor
- RIPA:
-
Radio-immunoprecipitation assay
- SC:
-
Satellite cell
- TTNPB:
-
Retinoic acid analogue
- 1A1:
-
Aldehyde dehydrogenase 1A1
- 1A3:
-
Aldehyde dehydrogenase 1A3
References
Bentzinger C, Florian W, Yu X, Rudnicki MA (2012) Building muscle: molecular regulation of myogenesis. In: Cold Spring Harbor perspectives in biology 4(2). https://doi.org/10.1101/cshperspect.a008342
Boya P, Codogno P, Rodriguez-Muela N (2018) Autophagy in stem cells: repair, remodelling and metabolic reprogramming. In: Development (Cambridge, England) 145(4). https://doi.org/10.1242/dev.146506
Calleja LF, Yoval-Sánchez B, Hernández-Esquivel L, Gallardo-Pérez JC, Sosa-Garrocho M, Marín-Hernández Á et al (2021) Activation of ALDH1A1 by omeprazole reduces cell oxidative stress damage. In: The FEBS J 288(13):S. 4064–4080. https://doi.org/10.1111/febs.15698
Carroll B, Otten EG, Manni D, Stefanatos R, Menzies FM, Smith GR et al (2018) Oxidation of SQSTM1/p62 mediates the link between redox state and protein homeostasis. In: Nat Commun 9(1):S. 256. https://doi.org/10.1038/s41467-017-02746-z
Cobb L (2013) Cell based assays: the cell cycle, cell proliferation and cell death. In: Mat Meth 3. https://doi.org/10.13070/mm.en.3.172
Collins SJ (2002) The role of retinoids and retinoic acid receptors in normal hematopoiesis. In: Leukemia 16(10):S. 1896–1905. https://doi.org/10.1038/sj.leu.2402718
Croker AK, Rodriguez-Torres M, Xia Y, Pardhan S, Leong HS, Lewis JD, Allan AL (2017) Differential functional roles of ALDH1A1 and ALDH1A3 in mediating metastatic behavior and therapy resistance of human breast cancer cells. In: Intern J Mole Sci 18(10). https://doi.org/10.3390/ijms18102039
Etienne J, Joanne P, Catelain C, Riveron S, Bayer AC, Lafable J et al (2020) Aldehyde dehydrogenases contribute to skeletal muscle homeostasis in healthy, aging, and Duchenne muscular dystrophy patients. In: Journal of Cachexia, Sarcopenia and Muscle. https://doi.org/10.1002/jcsm.12557
Fiacco E, Castagnetti F, Bianconi V, Madaro L, de Bardi M, Nazio F et al (2016) Autophagy regulates satellite cell ability to regenerate normal and dystrophic muscles. In: Cell Death Differ 23(11):S. 1839–1849. https://doi.org/10.1038/cdd.2016.70
Forcina L, Miano C, Pelosi L, Musarò A (2019) An overview about the biology of skeletal muscle satellite cells. In: Curr Genom 20(1):S. 24–37. https://doi.org/10.2174/1389202920666190116094736
Fortini P, Ferretti C, Iorio E, Cagnin M, Garribba L, Pietraforte D et al (2016) The fine tuning of metabolism, autophagy and differentiation during in vitro myogenesis. In: Cell Death Dis 7(3):e2168. https://doi.org/10.1038/cddis.2016.50
Gudas LJ (2012) Emerging roles for retinoids in regeneration and differentiation in normal and disease states. In: Biochimica et biophysica acta 1821(1):S. 213–221. https://doi.org/10.1016/j.bbalip.2011.08.002
Huang R, Xu Y, Wan W, Shou X, Qian J, You Z et al (2015) Deacetylation of nuclear LC3 drives autophagy initiation under starvation. In: Mole Cell 57(3):S. 456–466. https://doi.org/10.1016/j.molcel.2014.12.013
Jean E, Laoudj-Chenivesse D, Notarnicola C, Rouger K, Serratrice N, Bonnieu A et al (2011) Aldehyde dehydrogenase activity promotes survival of human muscle precursor cells. In: J Cell Mole Med 15(1):S. 119–133. https://doi.org/10.1111/j.1582-4934.2009.00942.x
Li J, Feng ZC, Yeung FS-H, Wong MR-M, Oakie A, Fellows GF et al (2014) Aldehyde dehydrogenase 1 activity in the developing human pancreas modulates retinoic acid signalling in mediating islet differentiation and survival. In: Diabetologia 57(4):S. 754–764. https://doi.org/10.1007/s00125-013-3147-y
Mukund K, Subramaniam S (2020) Skeletal muscle: a review of molecular structure and function, in health and disease. In: Wiley interdisciplinary reviews. Syst Biol Med 12(1):e1462. https://doi.org/10.1002/wsbm.1462
Papaconstantinou J, Wang CZ, Zhang M, Yang S, Deford J, Bulavin DV, Ansari NH (2015) Attenuation of p38α MAPK stress response signaling delays the in vivo aging of skeletal muscle myofibers and progenitor cells. In: Aging 7(9). https://doi.org/10.18632/aging.100802
Pequerul R, Vera J, Giménez-Dejoz J, Crespo I, Coines J, Porté S et al (2020) Structural and kinetic features of aldehyde dehydrogenase 1A (ALDH1A) subfamily members, cancer stem cell markers active in retinoic acid biosynthesis. In: Arch Biochem Biophysics 681:S. 108256. https://doi.org/10.1016/j.abb.2020.108256
Poturnajova M, Kozovska Z, Matuskova M (2021) Aldehyde dehydrogenase 1A1 and 1A3 isoforms - mechanism of activation and regulation in cancer. In: Cell Signal 87:S. 110120. https://doi.org/10.1016/j.cellsig.2021.110120
Pownall M, Elizabeth; Gustafsson, Marcus K., Emerson, Charles P. (2002) Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu Rev Cell Dev Biol 18:747–783. https://doi.org/10.1146/annurev.cellbio.18.012502.105758
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. In: Nat Protoc 8(11):S. 2281–2308. https://doi.org/10.1038/nprot.2013.143
Rihani L, Liesche-Starnecker F, Schlegel J (2021) Human skeletal muscle satellite cells co-express aldehyde dehydrogenase isoforms ALDH1A1 & ALDH1A3. In: J Cytol Histol (12):Artikel 568
Robinson DCL, Dilworth FJ (2018) Epigenetic regulation of adult myogenesis. Curr Top Dev Biol 126:235–284. https://doi.org/10.1016/bs.ctdb.2017.08.002
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. In: Curr Biol 24(10):R453-R462. https://doi.org/10.1016/j.cub.2014.03.034
Shen X, Collier JM, Hlaing M, Zhang L et al (2003) Genome-wide examination of myoblast cell cycle withdrawal during differentiation. In: Developmental dynamics : an official publication of the American Association of Anatomists 226(1):S. 128–138. https://doi.org/10.1002/dvdy.10200
Sin TK, Pei XM, Teng BT, Tam EW, Yung BY, Siu PM (2013) Oxidative stress and DNA damage signalling in skeletal muscle in pressure-induced deep tissue injury. In: Pflugers Archiv : Euro J Physiol 465(2). https://doi.org/10.1007/s00424-012-1205-9
Soprano DRT, Bryan W, Soprano KJ (2007) Role of retinoic acid in the differentiation of embryonal carcinoma and embryonic stem cells. In: Gerald Litwack (Hg.): vitamin A. Vitamins and hormones advances in research and applications, Bd. 75. Amsterdam: Elsevier, Acad Press (Vitamins and hormones, 75.2007):S. 69–95
Vassalli, G (2019) Aldehyde dehydrogenases: not just markers, but functional regulators of stem cells. In: Stem cells international 2019, S. 3904645. https://doi.org/10.1155/2019/3904645
Vauchez, Karine; Marolleau, Jean-Pierre; Schmid, Michel; Khattar, Patricia; Chapel, Alain; Catelain, Cyril et al. (2009): Aldehyde dehydrogenase activity identifies a population of human skeletal muscle cells with high myogenic capacities. In: Mole Therap 17(11):S. 1948–1958. https://doi.org/10.1038/mt.2009.204
Vella JB, Thompson SD, Bucsek MJ, Song M, Huard J (2011) Murine and human myogenic cells identified by elevated aldehyde dehydrogenase activity: implications for muscle regeneration and repair. In: PloS One 6(12):e29226. https://doi.org/10.1371/journal.pone.0029226
Wang, Chao; Kane, Maureen A.; Napoli, Joseph L. (2010): Multiple retinol and retinal dehydrogenases catalyze all-trans-retinoic acid biosynthesis in astrocytes*. In: The J Biol Chem 286(8):S. 6542–6553. https://doi.org/10.1074/jbc.M110.198382
Wang S, Zhao X, Liu Q, Wang Y, Li S, Xu S (2022) Selenoprotein K protects skeletal muscle from damage and is required for satellite cells-mediated myogenic differentiation. In: Redox Biol 50:S. 102255. https://doi.org/10.1016/j.redox.2022.102255
Wen X, Klionsky DJ (2016) Autophagy is a key factor in maintaining the regenerative capacity of muscle stem cells by promoting quiescence and preventing senescence. In: Autophagy 12(4):S. 617–618. https://doi.org/10.1080/15548627.2016.1158373
Wu M, Zhang X, Zhang W, Chiou YS, Qian W, Liu X et al (2022) Cancer stem cell regulated phenotypic plasticity protects metastasized cancer cells from ferroptosis. In: Nat Commun 13(1):S. 1371. https://doi.org/10.1038/s41467-022-29018-9
Wu W, Schecker J, Würstle S, Schneider F, Schönfelder M, Schlegel J (2018) Aldehyde dehydrogenase 1A3 (ALDH1A3) is regulated by autophagy in human glioblastoma cells. Cancer Lett 417:112–123. https://doi.org/10.1016/j.canlet.2017.12.036
Wu W, Wu Y, Mayer K, Rosenstiel CV, Schecker J, Baur S et al (2020) Lipid peroxidation plays an important role in chemotherapeutic effects of temozolomide and the development of therapy resistance in human glioblastoma. In: Trans Oncol 13(3):S. 100748. https://doi.org/10.1016/j.tranon.2020.100748
Acknowledgements
We would like to thank E. Beck, S. Baur, Ch. Schustetter, and T. Matt for their excellent technical assistance. We also would like to thank Kerstin Stemmer for the helpful discussion.
Author information
Authors and Affiliations
Contributions
LS and FK equally performed cell cultivation, experimental implementation, data collection, and analysis, with support from SF, WW, JS, and MK. The first draft of the manuscript was written by LS. All authors commented on previous versions of the manuscript, contributed to the study conception and design, and read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Preprint version
The manuscript was already submitted as a preprint (Research Square) with the corresponding author’s former last name Rihani. This updated version contains additional results and involves further theories.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Steingruber, L., Krabichler, F., Franzmeier, S. et al. ALDH1A1 and ALDH1A3 paralogues of aldehyde dehydrogenase 1 control myogenic differentiation of skeletal muscle satellite cells by retinoic acid-dependent and -independent mechanisms. Cell Tissue Res 394, 515–528 (2023). https://doi.org/10.1007/s00441-023-03838-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-023-03838-7