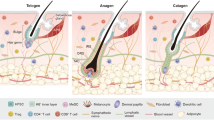
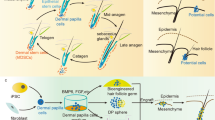
Abstract
Hair follicles are essential appendages of the mammalian skin, as hair performs vital functions of protection, thermoregulation and sensation. Hair follicles harbour exceptional regenerative abilities as they contain multiple somatic stem cell populations such as hair follicle stem cells (HFSCs) and melanocyte stem cells. Surrounding the stem cells and their progeny, diverse groups of cells and extracellular matrix proteins are organized to form a microenvironment (called ‘niche’) that serves to promote and maintain the optimal functioning of these stem cell populations. Recent studies have shed light on the intricate nature of the HFSC niche and its crucial role in regulating hair follicle regeneration. In this Review, we describe how the niche serves as a signalling hub, communicating, deciphering and integrating both local signals within the skin and systemic inputs from the body and environment to modulate HFSC activity. We delve into the recent advancements in identifying the cellular and molecular nature of the niche, providing a holistic perspective on its essential functions in hair follicle morphogenesis, regeneration and ageing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Morris, R. J. & Potten, C. S. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J. Invest. Dermatol. 112, 470–475 (1999).
Müller-Röver, S. et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 117, 3–15 (2001).
Greco, V. et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4, 155–169 (2009).
Paus, R. & Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 341, 491–497 (1999).
Rendl, M., Polak, L. & Fuchs, E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 22, 543–557 (2008).
Hsu, Y.-C., Pasolli, H. A. & Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144, 92–105 (2011).
Chase, H. B. Growth of the hair. Physiol. Rev. 34, 113–126 (1954).
Craven, A. J. et al. Prolactin delays hair regrowth in mice. J. Endocrinol. 191, 415–425 (2006).
Goldberg, L. J. & Lenzy, Y. Nutrition and hair. Clin. Dermatol. 28, 412–419 (2010).
Goldstein, J. et al. Calcineurin/Nfatc1 signaling links skin stem cell quiescence to hormonal signaling during pregnancy and lactation. Genes Dev. 28, 983–994 (2014).
Shwartz, Y. et al. Cell types promoting goosebumps form a niche to regulate hair follicle stem cells. Cell 182, 578–593.e19 (2020).
Zhang, B. et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 577, 676–681 (2020).
Cotsarelis, G., Sun, T.-T. & Lavker, R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990).
Tumbar, T. et al. Defining the epithelial stem cell niche in skin. Science 303, 359–363 (2004).
Blanpain, C., Lowry, W. E., Geoghegan, A., Polak, L. & Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648 (2004).
Claudinot, S., Nicolas, M., Oshima, H., Rochat, A. & Barrandon, Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc. Natl Acad. Sci. USA 102, 14677–14682 (2005).
Ito, M. et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 11, 1351–1354 (2005).
Matsumura, H. et al. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science 351, aad4395 (2016).
Driskell, R. R. et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504, 277–281 (2013).
Driskell, R. R. & Watt, F. M. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 25, 92–99 (2015).
Festa, E. et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146, 761–771 (2011).
Rahmani, W., Sinha, S. & Biernaskie, J. Immune modulation of hair follicle regeneration. NPJ Regen. Med. 5, 9 (2020).
Li, K. N. et al. Skin vasculature and hair follicle cross-talking associated with stem cell activation and tissue homeostasis. eLife 8, e45977 (2019).
Heitman, N. et al. Dermal sheath contraction powers stem cell niche relocation during hair cycle regression. Science 367, 161–166 (2020).
Kobielak, K., Pasolli, H. A., Alonso, L., Polak, L. & Fuchs, E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J. Cell Biol. 163, 609–623 (2003).
Nowak, J. A., Polak, L., Pasolli, H. A. & Fuchs, E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 3, 33–43 (2008).
Woo, W.-M., Zhen, H. H. & Oro, A. E. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin–Shh regulatory loop. Genes Dev. 26, 1235–1246 (2012).
Millar, S. E. Molecular mechanisms regulating hair follicle development. J. Invest. Dermatol. 118, 216–225 (2002).
Sennett, R. & Rendl, M. Mesenchymal–epithelial interactions during hair follicle morphogenesis and cycling. Semin. Cell Dev. Biol. 23, 917–927 (2012).
Hardy, M. H. The secret life of the hair follicle. Trends Genet. 8, 55–61 (1992).
Xu, Z. et al. Embryonic attenuated Wnt/β-catenin signaling defines niche location and long-term stem cell fate in hair follicle. eLife 4, e10567 (2015).
Saxena, N., Mok, K.-W. & Rendl, M. An updated classification of hair follicle morphogenesis. Exp. Dermatol. 28, 332–344 (2019).
Schmidt-Ullrich, R. & Paus, R. Molecular principles of hair follicle induction and morphogenesis. BioEssays 27, 247–261 (2005).
Ouspenskaia, T., Matos, I., Mertz, A. F., Fiore, V. F. & Fuchs, E. WNT-SHH antagonism specifies and expands stem cell prior. to niche formation. Cell 164, 156–169 (2016).
Morita, R. et al. Tracing the origin of hair follicle stem cells. Nature 594, 547–552 (2021).
Morris, R. J. et al. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 22, 411 (2004).
Oliver, R. F. The induction of hair follicle formation in the adult hooded rat by vibrissa dermal papillae. J. Embryol. Exp. Morphol. 23, 219–236 (1970).
Jahoda, Ca. B., Horne, K. A. & Oliver, R. F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature 311, 560–562 (1984).
Paus, R. Principles of hair cycle control. J. Dermatol. 25, 793–802 (1998).
Kobielak, K., Stokes, N., Cruz, J., de la, Polak, L. & Fuchs, E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc. Natl Acad. Sci. USA 104, 10063–10068 (2007).
Plikus, M. V. et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451, 340–344 (2008).
Horsley, V., Aliprantis, A. O., Polak, L., Glimcher, L. H. & Fuchs, E. NFATc1 balances quiescence and proliferation of skin stem. Cells Cell 132, 299–310 (2008).
Oshimori, N. & Fuchs, E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 10, 63–75 (2012).
Morgan, B. A. The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb. Perspect. Med. 4, a015180 (2014).
Rompolas, P. et al. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 487, 496–499 (2012).
Foitzik, K. et al. Control of murine hair follicle regression (catagen) by TGF-β1 in vivo. FASEB J. 14, 752–760 (2000).
Mesa, K. R. et al. Niche-induced cell death and epithelial phagocytosis regulate hair follicle stem cell pool. Nature 522, 94–97 (2015).
Harshuk-Shabso, S., Dressler, H., Niehrs, C., Aamar, E. & Enshell-Seijffers, D. Fgf and Wnt signaling interaction in the mesenchymal niche regulates the murine hair cycle clock. Nat. Commun. 11, 5114 (2020).
Rahmani, W. et al. Hair follicle dermal stem cells regenerate the dermal sheath, repopulate the dermal papilla, and modulate hair type. Dev. Cell 31, 543–558 (2014).
Martino, P. A., Heitman, N. & Rendl, M. The dermal sheath: an emerging component of the hair follicle stem cell niche. Exp. Dermatol. 30, 512–521 (2021).
Martino, P. et al. Progenitor-derived endothelin controls dermal sheath contraction for hair follicle regression. Nat. Cell Biol. 25, 222–234 (2023).
Rodeheffer, M. S., Birsoy, K. & Friedman, J. M. Identification of white adipocyte progenitor cell vivo. Cell 135, 240–249 (2008).
Cristancho, A. G. & Lazar, M. A. Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 12, 722–734 (2011).
Rivera-Gonzalez, G. C. et al. Skin adipocyte stem cell self-renewal is regulated by a PDGFA/AKT-signaling axis. Cell Stem Cell 19, 738–751 (2016).
Keyes, B. E. et al. Nfatc1 orchestrates aging in hair follicle stem cells. Proc. Natl Acad. Sci. USA 110, E4950–E4959 (2013).
Hsu, Y.-C., Li, L. & Fuchs, E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 157, 935–949 (2014).
Zhang, B. & Hsu, Y.-C. Emerging roles of transit-amplifying cells in tissue regeneration and cancer. Wiley Interdiscip. Rev. Dev. Biol. 6, 10.1002/wdev.282 (2017).
Kimura-Ueki, M. et al. Hair cycle resting phase is regulated by cyclic epithelial FGF18 signaling. J. Invest. Dermatol. 132, 1338–1345 (2012).
Perdigoto, C. N. et al. Polycomb-mediated repression and sonic hedgehog signaling interact to regulate merkel cell specification during skin development. PLoS Genet. 12, e1006151 (2016).
Fujiwara, H. et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144, 577–589 (2011).
Botchkarev, V. A., Botchkareva, N. V., Peters, E. M. & Paus, R. Epithelial growth control by neurotrophins: leads and lessons from the hair follicle. Prog. Brain Res. 146, 493–513 (2004).
Rutlin, M. et al. The cellular and molecular basis of direction selectivity of Aδ-LTMRs. Cell 159, 1640–1651 (2014).
Cheng, C.-C. et al. Hair follicle epidermal stem cells define a niche for tactile sensation. eLife 7, e3883 (2018).
Peng, J., Chen, H. & Zhang, B. Nerve–stem cell crosstalk in skin regeneration and diseases. Trends Mol. Med. 28, 583–595 (2022).
Li, K. N. & Tumbar, T. Hair follicle stem cells as a skin-organizing signaling center during adult homeostasis. EMBO J. 40, e107135 (2021).
Zhang, B. et al. Hair follicles’ transit-amplifying cells govern concurrent dermal adipocyte production through Sonic Hedgehog. Genes. Dev. 30, 2325–2338 (2016).
Durward, A. & Rudall, K. M. in The Biology of Hair Growth (eds Montagna, W. & Ellis, R. A.) ch. 9 189–218 (Academic Press, 1958).
Moretti, G., Ellis, R. A. & Mescon, H. Vascular patterns in the skin of the face. J. Invest. Dermatol. 33, 103–112 (1959).
Skobe, M. & Detmar, M. Structure, function, and molecular control of the skin lymphatic system. J. Investig. Dermatol. Symp. Proc. 5, 14–19 (2000).
Kam, C. Y. et al. Mechanisms of skin vascular maturation and maintenance captured by longitudinal imaging of live mice. Cell 186, 2345–2360.e16 (2023).
Li, K. N., Chovatiya, G., Ko, D. Y., Sureshbabu, S. & Tumbar, T. Blood endothelial ALK1–BMP4 signaling axis regulates adult hair follicle stem cell activation. EMBO J. 42, e112196 (2023).
Braverman, I. M. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J. Invest. Dermatol. 93, S2–S9 (1989).
Gay, D. & Ito, M. The seed tends to the soil: hair follicle stem cells remodel their lymphatic niche. Cell Stem Cell 25, 733–734 (2019).
Peña-Jimenez, D. et al. Lymphatic vessels interact dynamically with the hair follicle stem cell niche during skin regeneration in vivo. EMBO J. 38, e101688 (2019).
Gur-Cohen, S. et al. Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 366, 1218–1225 (2019).
Di Meglio, P., Perera, G. K. & Nestle, F. O. The multitasking organ: recent insights into skin immune function. Immunity 35, 857–869 (2011).
Quaresma, J. A. S. Organization of the skin immune system and compartmentalized immune responses in infectious diseases. Clin. Microbiol. Rev. 32, e00034-18 (2019).
Lay, K. et al. Stem cells repurpose proliferation to contain a breach in their niche barrier. eLife 7, e41661 (2018).
Paus, R., Nickoloff, B. J. & Ito, T. A ‘hairy’ privilege. Trends Immunol. 26, 32–40 (2005).
Castellana, D., Paus, R. & Perez-Moreno, M. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLOS Biol. 12, e1002002 (2014).
Wang, E. C. E., Dai, Z., Ferrante, A. W., Drake, C. G. & Christiano, A. M. A subset of TREM2+ dermal macrophages secretes oncostatin M to maintain hair follicle stem cell quiescence and inhibit hair growth. Cell Stem Cell 24, 654–669.e6 (2019).
Ali, N. et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 169, 1119–1129.e11 (2017).
Ali, N. & Rosenblum, M. D. Regulatory T cells in skin. Immunology 152, 372–381 (2017).
Fraser, A. S., Nay, T. & Turner, H. N. Growth of the mouse coat. II. Effect of sex and pregnancy. Aust. J. Biol. Sci. 6, 645–656 (1953).
Movérare, S., Lindberg, M. K., Ohlsson, C., Faergemann, J. & Gustafsson, J.-Å. Estrogen receptor α, but not estrogen receptor β, is involved in the regulation of the hair follicle cycling as well as the thickness of epidermis in male mice. J. Invest. Dermatol. 119, 1053–1058 (2002).
Osthaus, B. et al. Hair coat properties of donkeys, mules and horses in a temperate climate. Equine Vet. J. 50, 339–342 (2018).
Ferreira, M. S. et al. Transcriptomic regulation of seasonal coat color change in hares. Ecol. Evol. 10, 1180–1192 (2020).
O’Brien, C., Darcy-Dunne, M. R. & Murphy, B. A. The effects of extended photoperiod and warmth on hair growth in ponies and horses at different times of year. PLoS ONE 15, e0227115 (2020).
Tietgen, L. et al. Fur colour in the Arctic fox: genetic architecture and consequences for fitness. Proc. R. Soc. B Biol. Sci. 288, 20211452 (2021).
Roman, K., Wilk, M., Książek, P., Czyż, K. & Roman, A. the effect of the season, the maintenance system and the addition of polyunsaturated fatty acids on selected biological and physicochemical features of rabbit fur. Animals 12, 971 (2022).
Aragona, M. et al. Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature 584, 268–273 (2020).
Xie, Y. et al. Hair shaft miniaturization causes stem cell depletion through mechanosensory signals mediated by a Piezo1-calcium-TNF-α axis. Cell Stem Cell 29, 70–85.e6 (2022).
Cotsarelis, G. & Millar, S. E. Towards a molecular understanding of hair loss and its treatment. Trends Mol. Med. 7, 293–301 (2001).
Lei, M. & Chuong, C.-M. Aging, alopecia, and stem cells. Science 351, 559–560 (2016).
Meacham, C. E., DeVilbiss, A. W. & Morrison, S. J. Metabolic regulation of somatic stem cells in vivo. Nat. Rev. Mol. Cell Biol. 23, 428–443 (2022).
Flores, A. et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat. Cell Biol. 19, 1017–1026 (2017).
Kim, C. S. et al. Glutamine metabolism controls stem cell fate reversibility and long-term maintenance in the hair follicle. Cell Metab. 32, 629–642.e8 (2020).
Karnik, P. et al. Hair follicle stem cell-specific PPARγ deletion causes scarring alopecia. J. Invest. Dermatol. 129, 1243–1257 (2009).
Deng, Z. et al. mTOR signaling promotes stem cell activation via counterbalancing BMP-mediated suppression during hair regeneration. J. Mol. Cell Biol. 7, 62–72 (2015).
Shapiro, J. Hair loss in women. N. Engl. J. Med. 357, 1620–1630 (2007).
Strumia, R. Eating disorders and the skin. Clin. Dermatol. 31, 80–85 (2013).
Guo, E. L. & Katta, R. Diet and hair loss: effects of nutrient deficiency and supplement use. Dermatol. Pract. Concept. 7, 1–10 (2017).
Morinaga, H. et al. Obesity accelerates hair thinning by stem cell-centric converging mechanisms. Nature 595, 266–271 (2021).
Paatela, E., Munson, D. & Kikyo, N. Circadian regulation in tissue regeneration. Int. J. Mol. Sci. 20, 2263 (2019).
Ruby, C. L., Major, R. J. & Hinrichsen, R. D. Regulation of tissue regeneration by the circadian clock. Eur. J. Neurosci. 53, 3576–3597 (2021).
Tanioka, M. et al. Molecular clocks in mouse skin. J. Invest. Dermatol. 129, 1225–1231 (2009).
Akashi, M. et al. Noninvasive method for assessing the human circadian clock using hair follicle cells. Proc. Natl Acad. Sci. USA 107, 15643–15648 (2010).
Al-Nuaimi, Y. et al. A meeting of two chronobiological systems: circadian proteins period1 and BMAL1 modulate the human hair cycle clock. J. Invest. Dermatol. 134, 610–619 (2014).
Janich, P. et al. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature 480, 209–214 (2011).
Lin, K. K. et al. Circadian clock genes contribute to the regulation of hair follicle cycling. PLOS Genet. 5, e1000573 (2009).
Geyfman, M. et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc. Natl Acad. Sci. USA 109, 11758–11763 (2012).
Plikus, M. V. et al. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc. Natl Acad. Sci. USA 110, E2106–E2115 (2013).
COMAISH, S. Autoradiographic studies of hair groawth in various dermatoses: investigation of a possible circadian rhythm in human hair growth. Br. J. Dermatol. 81, 283–288 (1969).
Roosterman, D., Goerge, T., Schneider, S. W., Bunnett, N. W. & Steinhoff, M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol. Rev. 86, 1309–1379 (2006).
Glatte, P., Buchmann, S. J., Hijazi, M. M., Illigens, B. M.-W. & Siepmann, T. Architecture of the cutaneous autonomic nervous system. Front. Neurol. 10, 970 (2019).
Li, L. et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147, 1615–1627 (2011).
Bai, L. et al. Genetic identification of an expansive mechanoreceptor sensitive to skin stroking. Cell 163, 1783–1795 (2015).
Furlan, A. et al. Visceral motor neuron diversity delineates a cellular basis for nipple- and pilo-erection muscle control. Nat. Neurosci. 19, 1331–1340 (2016).
Brownell, I., Guevara, E., Bai, C. B., Loomis, C. A. & Joyner, A. L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8, 552–565 (2011).
Fan, S. M.-Y. et al. External light activates hair follicle stem cells through eyes via an ipRGC–SCN–sympathetic neural pathway. Proc. Natl Acad. Sci. USA 115, E6880–E6889 (2018).
Brunet, A., Goodell, M. A. & Rando, T. A. Ageing and rejuvenation of tissue stem cells and their niches. Nat. Rev. Mol. Cell Biol. 24, 45–62 (2022).
Ogrodnik, M. & Gladyshev, V. N. The meaning of adaptation in aging: insights from cellular senescence, epigenetic clocks and stem cell alterations. Nat. Aging 3, 766–775 (2023).
Colavincenzo, M. L. & Granstein, R. D. Stress and the skin: a meeting report of the weill cornell symposium on the science of dermatology. J. Invest. Dermatol. 126, 2560–2561 (2006).
Sawaya, M. E. & Hordinsky, M. K. glucocorticoid regulation of hair growth in alopecia areata. J. Invest. Dermatol. 104, 30 (1995).
Jang, H., Jo, Y., Lee, J. H. & Choi, S. Aging of hair follicle stem cells and their niches. BMB Rep. 56, 2–9 (2023).
Steptoe, A. & Kivimäki, M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 9, 360–370 (2012).
Qin, H.-Y., Cheng, C.-W., Tang, X.-D. & Bian, Z.-X. Impact of psychological stress on irritable bowel syndrome. World J. Gastroenterol. 20, 14126–14131 (2014).
Rosenberg, S. L., Miller, G. E., Brehm, J. M. & Celedón, J. C. Stress and asthma: novel insights on genetic, epigenetic and immunologic mechanisms. J. Allergy Clin. Immunol. 134, 1009–1015 (2014).
Navarini, A. A. & Nobbe, S. Marie Antoinette syndrome. Arch. Dermatol. 145, 656–656 (2009).
Arck, P. C., Slominski, A., Theoharides, T. C., Peters, E. M. J. & Paus, R. Neuroimmunology of stress: skin takes center stage. J. Invest. Dermatol. 126, 1697–1704 (2006).
Condamina, M. et al. Factors associated with perceived stress in patients with vitiligo in the ComPaRe e-cohort. J. Am. Acad. Dermatol. 86, 696–698 (2022).
Choi, S. et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 592, 428–432 (2021).
Rabbani, P. et al. Coordinated activation of wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 145, 941–955 (2011).
Rachmin, I. et al. Stress-associated ectopic differentiation of melanocyte stem cells and ORS amelanotic melanocytes in an ex vivo human hair follicle model. Exp. Dermatol. 30, 578–587 (2021).
Lerner, A. B. Gray hair and sympathectomy. Report of a case. Arch. Dermatol. 93, 235–236 (1966).
Ortonne, J. P., Thivolet, J. & Guillet, R. Graying of hair with age and sympathectomy. Arch. Dermatol. 118, 876–877 (1982).
Fernandez-Flores, A., Saeb-Lima, M. & Cassarino, D. S. Histopathology of aging of the hair follicle. J. Cutan. Pathol. 46, 508–519 (2019).
Williams, R., Pawlus, A. D. & Thornton, M. J. Getting under the skin of hair aging: the impact of the hair follicle environment. Exp. Dermatol. 29, 588–597 (2020).
Nishimura, E. K., Granter, S. R. & Fisher, D. E. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science 307, 720–724 (2005).
Keyes, B. E. & Fuchs, E. Stem cells: aging and transcriptional fingerprints. J. Cell Biol. 217, 79–92 (2017).
Zhang, S. & Duan, E. Fighting against skin aging. Cell Transpl. 27, 729–738 (2018).
Doles, J., Storer, M., Cozzuto, L., Roma, G. & Keyes, W. M. Age-associated inflammation inhibits epidermal stem cell function. Genes. Dev. 26, 2144–2153 (2012).
Ge, Y. et al. The aging skin microenvironment dictates stem cell behavior. Proc. Natl Acad. Sci. USA 117, 5339–5350 (2020).
Zhang, C. et al. Escape of hair follicle stem cells causes stem cell exhaustion during aging. Nat. Aging 1, 889–903 (2021).
Lay, K., Kume, T. & Fuchs, E. FOXC1 maintains the hair follicle stem cell niche and governs stem cell quiescence to preserve long-term tissue-regenerating potential. Proc. Natl Acad. Sci. USA 113, E1506–E1515 (2016).
Li, G. et al. SIRT7 activates quiescent hair follicle stem cells to ensure hair growth in mice. EMBO J. 39, e104365 (2020).
Barrandon, Y. & Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl Acad. Sci. USA 84, 2302–2306 (1987).
Koester, J. et al. Niche stiffening compromises hair follicle stem cell potential during ageing by reducing bivalent promoter accessibility. Nat. Cell Biol. 23, 771–781 (2021).
Branchet, M. C., Boisnic, S., Frances, C., Lesty, C. & Robert, L. Morphometric analysis of dermal collagen fibers in normal human skin as a function of age. Arch. Gerontol. Geriatr. 13, 1–14 (1991).
Farage, M. A., Miller, K. W., Elsner, P. & Maibach, H. I. Characteristics of the aging skin. Adv. Wound Care 2, 5–10 (2013).
Duncan, K. O. & Leffell, D. J. Preoperative assessment of the elderly patient. Dermatol. Clin. 15, 583–593 (1997).
Salzer, M. C. et al. Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell 175, 1575–1590.e22 (2018).
Mine, S., Fortunel, N. O., Pageon, H. & Asselineau, D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: a new view of skin morphogenesis and aging. PLoS ONE 3, e4066 (2008).
Shin, W. et al. Dysfunction of hair follicle mesenchymal progenitors contributes to age-associated hair loss. Dev. Cell 53, 185–198.e7 (2020).
Rodriguez, R. S. et al. Memory regulatory T cells reside in human skin. J. Clin. Invest. 124, 1027–1036 (2014).
Liu, Z. et al. Glucocorticoid signaling and regulatory T cells cooperate to maintain the hair-follicle stem-cell niche. Nat. Immunol. 23, 1086–1097 (2022).
Tanimura, S. et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell 8, 177–187 (2011).
Chang, C.-Y. et al. NFIB is a governor of epithelial–melanocyte stem cell behaviour in a shared niche. Nature 495, 98–102 (2013).
Lu, Z. et al. Hair follicle stem cells regulate retinoid metabolism to maintain the self-renewal niche for melanocyte stem cells. eLife 9, e52712 (2020).
Sun, Q. et al. Dedifferentiation maintains melanocyte stem cells in a dynamic niche. Nature 616, 774–782 (2023).
Rompolas, P., Mesa, K. R. & Greco, V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 502, 513–518 (2013).
Geueke, A. et al. The anti-apoptotic Bcl-2 protein regulates hair follicle stem cell function. EMBO Rep. 22, e52301 (2021).
Rognoni, E. & Watt, F. M. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol. 28, 709–722 (2018).
Gurtner, G. C., Werner, S., Barrandon, Y. & Longaker, M. T. Wound repair and regeneration. Nature 453, 314–321 (2008).
Sen, C. K. et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair. Regen. 17, 763–771 (2009).
Ito, M. et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320 (2007).
Plikus, M. V. et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 355, 748–752 (2017).
Rinkevich, Y. et al. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348, aaa2151 (2015).
Jiang, D. et al. Two succeeding fibroblastic lineages drive dermal development and the transition from regeneration to scarring. Nat. Cell Biol. 20, 422–431 (2018).
Correa-Gallegos, D. et al. Patch repair of deep wounds by mobilized fascia. Nature 576, 287–292 (2019).
Guerrero-Juarez, C. F. et al. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat. Commun. 10, 650 (2019).
Mascharak, S. et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 372, eaba2374 (2021).
Wang, Q. et al. A multi-scale model for hair follicles reveals heterogeneous domains driving rapid spatiotemporal hair growth patterning. eLife 6, e22772 (2017).
Yu, Z. et al. Hoxc-dependent mesenchymal niche heterogeneity drives regional hair follicle regeneration. Cell Stem Cell 23, 487–500.e6 (2018).
Chang, H. Y. et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl Acad. Sci. USA 99, 12877–12882 (2002).
Xu, Z. et al. Anatomically distinct fibroblast subsets determine skin autoimmune patterns. Nature 601, 118–124 (2022).
Picardo, M. et al. Vitiligo. Nat. Rev. Dis. Prim. 1, 15011 (2015).
Acknowledgements
The authors thank Y.-C. Hsu, J. Peng, C. Wang and P. Zhang for discussion and critical feedback of the manuscript; J. Peng for designing the illustrations and Y. Xie for help with the revision. The authors regret that they were not able to discuss all the relevant works here owing to space constraints. This work was supported in part by grants from the National Natural Science Foundation of China (Project 32170850 to B.Z., Projects 32070873 and 32225018 to T.C.), Ministry of Science and Technology of China (Projects 2021YFA1101000 and 2022YFA0807300 to T.C.), Westlake University Education Foundation and Westlake Laboratory of Life Sciences and Biomedicine.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Rui Yi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Chen, T. Local and systemic mechanisms that control the hair follicle stem cell niche. Nat Rev Mol Cell Biol 25, 87–100 (2024). https://doi.org/10.1038/s41580-023-00662-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-023-00662-3