Abstract
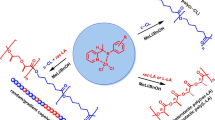
The dimeric amide lanthanum complex {[\({\text{Pzl}}_{{\text{2}}}^{{{\text{Me2}}}}\)CP(O)Ph2]La[N(SiMe3)2](µ2-OP(O)Ph2)}2 (PzlMe2 is 3,5-dimethylpyrazole) bearing the N,N,O-tridentate heteroscorpionate ligand is synthesized. As found by X-ray diffraction (XRD) (CIF file CCDC no. 2212274), the complex is binuclear and its lanthanum ions are linked by two bridging monoanionic diphenyl phosphinate ligands. The synthesized lanthanum complex demonstrates a high catalytic activity in the polymerization with ring opening of rac-lactide and ε-caprolactone providing the quantitative conversion of 500 equivalents of the monomer to the polymer at room temperature within 360–720 min for rac-lactide and 10–30 min for ε-caprolactone. The formed polylactides are characterized by the atactic microstructure (Pr = 0.54–0.56) and polydispersity indices (PDI) of 1.6–2.5, whereas for polycaprolactone PDI = 2.1–2.8.

Similar content being viewed by others
REFERENCES
Hou, Z. and Nishiura, M., Nat. Chem., 2010, vol. 2, p. 257. https://doi.org/10.1038/nchem.595
Trifonov, A.A. and Lyubov, D.M., Coord. Chem. Rev., 2017, vol. 340, p. 10. https://doi.org/10.1016/j.ccr.2016.09.013
Carpentier, J.-F., Gromada, J., and Mortreux, A., Coord. Chem. Rev., 2004, vol. 248, p. 397. https://doi.org/10.1016/j.ccr.2004.02.002
Friebe, O.N., Obrecht, W., and Zimmermann, M., Adv. Polym. Sci., 2006, vol. 204, p. 1. https://doi.org/10.1007/12_094
Anwander, R., Törnroos, K.W., and Zimmermann, M., Angew. Chem., Int. Ed. Engl., 2008, vol. 47, p. 775. https://doi.org/10.1002/anie.200703514
Cui, D., Liu, B., Wang, B., et al., Struct. Bond., 2010, vol. 137, p. 49. https://doi.org/10.1007/430.2010.16
Cotton, S.A., Coord. Chem. Rev., 1997, vol. 160, p. 93. https://doi.org/10.1016/S0010-8545(96)01340-9
Lyubov, D.M., Tolpygin, A.O., and Trifonov, A.A., Coord. Chem. Rev., 2019, vol. 392, p. 83. https://doi.org/10.1016/j.ccr.2019.04.013
Aubrecht, K.B., Chang, K., Hillmyer, M.A., and Tolman, W.B., J. Polym. Sci., Part A, 2001, vol. 39, p. 284. https://doi.org/10.1002/1099-0518(20010115)39
Tolpygin, A.O., Linnikova, O.A., Glukhova, T.A., et al., RSC Adv., 2016, vol. 6, p. 17913. https://doi.org/10.1039/C5RA27960G
Nakayama, Y. and Yasuda, H., J. Organomet. Chem., 2004, vol. 689, p. 4489. https://doi.org/10.1016/j.jorganchem.2004.05.056
Piers, W.E. and Emslie, D.J.H., Coord. Chem. Rev., 2002, vols. 233–234, p. 131. https://doi.org/10.1016/S0010-8545(02)00016-4
Howe, R.G., Tredget, C.S., Lawrence, S.C., et al., Chem. Commun., 2006, p. 223. https://doi.org/10.1039/B513927A
Zeimentz, P.M., Arndt, S., Elvidge, B.R., and Okuda, J., Chem. Rev., 2006, vol. 106, p. 2404. https://doi.org/10.1021/cr050574s
Hou, Z., Luo, Y., and Li, X., J. Organomet. Chem., 2006, vol. 691, p. 3114. https://doi.org/10.1016/j.jorganchem.2006.01.055
Molander, G.A. and Romero, J.A.C., Chem. Rev., 2002, vol. 102, p. 2161. https://doi.org/10.1021/cr010291+
Trifonov, A.A., Basalov, I.V., and Kissel, A.A., Dalton Trans., 2016, vol. 45, p. 19172. https://doi.org/10.1039/C6DT03913H
Kissel, A.A., Lyubov, D.M., Mahrova, T.V., et al., Dalton Trans., 2013, vol. 42, p. 9211. https://doi.org/10.1039/C3DT33108C
Khristolyubov, D.O., Lyubov, D.M., and Trifonov, A.A., Russ. Chem. Rev., 2021, vol. 90, p. 529. https://doi.org/10.1070/RCR4992
Shannon, R.D., Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, vol. 32, p. 751. https://doi.org/10.1107/S0567739476001551
Jia, Y.Q., J. Solid State Chem., 1991, vol. 95, p. 184. https://doi.org/10.1016/0022-4596(91)90388-x
Morss, L.R., Chem. Rev., 1976, vol. 76, p. 827. https://doi.org/10.1021/cr60304a007
Trifonov, A.A., Coord. Chem. Rev., 2010, vol. 254, p. 1327. https://doi.org/10.1016/j.ccr.2010.01.008
Otero, A., Lara-Sanchez, A., Castro-Osma, J.A., et al., New J. Chem., 2015, vol. 39, p. 7672. https://doi.org/10.1039/C5NJ00930H
Bochkarev, M.N., Zakharov, L.N., and Kalinina, G.S., Top Organomet. Chem., 1999, p. 285.
Barker, J. and Kilner, M., Coord. Chem. Rev., 1994, vol. 133, p. 219. https://doi.org/10.1016/0010-8545(94)80059-6
Trifonov, A.A., Russ. Chem. Rev., 2007, vol. 76, p. 1051. https://doi.org/10.1070/RC2007v076n11ABEH003693
Yap, G.P.A., Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2013, vol. 69, p. 937. https://doi.org/10.1107/S0108270113019902
Trofimenko, S., Scorpionates: The Coordination Chemistry of Polypyrazolylborate Ligands, London: Imperial College, 1998.
Reger, D.L., Comments Inorg. Chem., 1999, vol. 21, p. 1. https://doi.org/10.1080/02603599908020413
Otero, A., Fernandez-Baeza, J., Antinolo, A., et al., Dalton Trans., 2004, p. 1499. https://doi.org/10.1039/B401425A
Pettinari, C. and Pettinari, R., Coord. Chem. Rev., 2005, vol. 249, p. 525. https://doi.org/10.1016/j.ccr.2004.05.010
Mou, Z., Liu, B., Liu, X., et al., Macromolecules, 2014, vol. 47, p. 2233. https://doi.org/10.1021/ma500209t
Ballard, D.G.H., Coutis, A., Holton, J., et al., Chem. Commun., 1978, p. 994. https://doi.org/10.1039/C39780000994
Burger, B.J., Thompson, M.E., Cotter, W.D., and Bercaw, J.E., J. Am. Chem. Soc., 1990, vol. 112, p. 1566. https://doi.org/10.1021/ja00160a041
Hou, Z., Zhang, Y., Nishiura, M., and Wakatsuki, Y., Organometallics, 2003, vol. 22, p. 129. https://doi.org/10.1021/om020742w
Li, X. and Hou, Z., Macromolecules, 2005, vol. 38, p. 6767. https://doi.org/10.1021/ma051323o
Otero, A., Lara-Sanchez, A., Nájera, C., et al., Organometallics, 2012, vol. 31, p. 2244. https://doi.org/10.1021/om2011672
Pettinari, C. and Pettinari, R., Coord. Chem. Rev., 2005, vol. 249, p. 663. https://doi.org/10.1016/j.ccr.2004.08.017
Otero, A., Fernández-Baeza, J., Antinolo, A., et al., J. Am. Chem. Soc., 2004, vol. 126, p. 1330. https://doi.org/10.1021/ja0391558
Schädle, D., Maichle-Mössmer, C., Schädle, C., and Anwander, R., Chem.-Eur. J., 2014, vol. 21, p. 662. https://doi.org/10.1002/chem.201404792
Marques, N., Sella, A., and Takats, J., Chem. Rev., 2002, vol. 102, p. 2137. https://doi.org/10.1021/cr010327y
Trofimenko, S., Polyhedron, 2004, vol. 23, p. 197. https://doi.org/10.1016/j.poly.2003.11.013
Bigmore, H.R., Lawrence, S.C., Mountford, P., and Tredget, C.S., Dalton Trans., 2005, p. 635. https://doi.org/10.1039/B413121E
Gibson, V.C. and Spitzmesser, S.K., Chem. Rev., 2003, vol. 103, p. 283. https://doi.org/10.1021/cr980461r
Martínez, J., Otero, A., Lara-Sánchez, A., et al., Organometallics, 2016, vol. 35, p. 1802. https://doi.org/10.1021/acs.organomet.6b00203
Bradley, D.C., Ghotra, J.S., and Hart, F.A., Dalton Trans., 1973, vol. 10, p. 1021. https://doi.org/10.1039/DT9730001021
Barakat, I., Dubois, P., Jerome, R., and Teyssie, P., J. Polym. Sci., Part A, 1993, vol. 31, p. 505. https://doi.org/10.1002/pola.1993.080310222
APEX3, Madison: Bruker AXS Inc., 2018.
Krause, L., Herbst-Irmer, R., Sheldrick, G.M., and Stalke, D., J. Appl. Crystallogr., 2015, vol. 48, p. 3. https://doi.org/10.1107/S1600576714022985
Sheldrick, G., Acta Crystallogr., Sect. C: Struct. Chem., 2015, vol. 71, p. 3. https://doi.org/10.1107/S2053229614024218
Krieck, S., Koch, A., Hinze, K., et al., Eur. J. Inorg. Chem., 2016, p. 2332. https://doi.org/10.1002/ejic.201501263
Wingerter, S., Pfeiffer, M., Baier, F., et al., Z. Anorg. Allg. Chem., 2000, vol. 626, p. 1121. https://doi.org/10.1002/(SICI)1521-3749(200005)626:5<1121::AID-ZAAC1121>3.0.CO;2-I
Beswick, M.A., Cromhout, N.L., Harmer, C.N., et al., Chem. Commun., 1997, p. 583. https://doi.org/10.1039/A608202E
Al-Shboul, T.M.A., Volland, G., Gorls, H., et al., Inorg. Chem., 2012, vol. 51, p. 7903. https://doi.org/10.1021/ic300975s
Zhang, Z., Xu, X., Li, W., et al., Inorg. Chem., 2009, vol. 48, p. 5715. https://doi.org/10.1021/ic802177y
Litlabo, R., Zimmermann, M., Saliu, K., et al., Angew. Chem., Int. Ed. Engl., 2008, vol. 47, p. 9560. https://doi.org/10.1002/anie.200803856
Dong, X. and Robinson, J.R., Chem. Sci., 2020, vol. 11, p. 8184. https://doi.org/10.1039/D0SC03507F
Sugiyama, H., Korobkov, I., and Gambarotta, S., Inorg. Chem., 2004, vol. 43, p. 5771. https://doi.org/10.1021/ic049820t
Gu, X.-Y., Han, X.-Z., Yao, Y.-M., et al., J. Organomet. Chem., 2010, vol. 695, p. 2726. https://doi.org/10.1016/j.jorganchem.2010.07.037
Zhang, J., Qiu, J., Yao, Y., et al., Organometallics, 2012, vol. 31, p. 3138. https://doi.org/10.1021/om300036a
ACKNOWLEDGMENTS
The workers of the Nesmeyanov Institute of Organoelement Compounds (Russian Academy of Sciences) are grateful to the Ministry of Science and Higher Education of the Russian Federation for financial support.
Funding
The XRD and NMR studies of the compounds were carried out using the equipment of the Center for Collective Use “Analytical Center of Institute of Organometallic Chemistry of Russian Academy of Sciences” and supported by the project “Provision of Development of Material Technical Infrastructure of Centers for Collective Use of Scientific Equipment” (unique identifier RF−2296.61321X0017, agreement no. 075-15-2021-670).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author of this work declares that they has no conflicts of interest.
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Rad’kova, N.Y., Cherkasov, A.V. & Trifonov, A.A. Synthesis and Structure of the (µ2-OP(O)Ph2)-Linked Dimeric Amide Lanthanum Complex {[\({\text{Pzl}}_{2}^{{{\text{Me2}}}}\)CP(O)Ph2]La[N(SiMe3)2](µ2-OP(O)Ph2)}2 Bearing the Tridentate Heteroscorpionate Ligand. Investigation of the Catalytic Activity in rac-Lactide and ε-Caprolactone Polymerization. Russ J Coord Chem 49, 710–717 (2023). https://doi.org/10.1134/S1070328423600717
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328423600717