Abstract
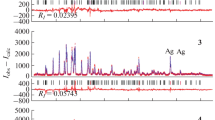
Solid-phase reactions of silver trifluoroacetate CF3COOAg with copper, indium, and zinc are studied by thermogravimetry, differential scanning calorimetry, and mass spectrometry. In a temperature range of 358–428 K, the reactions are found to afford trifluoroacetates of these metals without mass loss of the weighed samples. The obtained experimental data make it possible to calculate the enthalpy of formation of copper trifluoroacetate \({{\Delta }_{f}}H_{{298}}^{^\circ }\)(CF3СООСu, cr) = –1020.5 ± 18.0 kJ/mol.


Similar content being viewed by others
REFERENCES
Syrkin, V.G. CVD-metod. Khimicheskaya parofaznaya metallizatsiya (CVD Method. Chemical Vapor Metallization), Moscow: Nauka, 2000.
Fromm, K.M. and Gueneau, E.D., Polyhedron, 2004, vol. 23, p. 1479.
Paramonov, S., Samoilenkov, S., Papucha, S., et al., J. Phys. IV, 2001, vol. 11, p. Pr3-645-52.
Morozova, E.A., Dobrokhotova, Zh.V., and Alikhanyan, A.S., J. Therm. Anal. Calorim., 2017, vol. 130, no. 3, p. 2211.
Lukyanova, V.A., Papina, T.S., Didenko, K.V., et al., J. Therm. Anal. Calorim., 2008, vol. 92, p. 743.
Kamkin, N.N., Kayumova, D.B., Yaryshev, N.G., et al., Russ. J. Inorg. Chem., 2012, vol. 57, p. 1308.
Luo, Y.-R., Handbook of Bond Dissociation Energies in Organic Compounds, CRC Press LLC, 2003.
Termicheskie konstanty veshchestv. Spravochnik (Thermal Characteristics of Substances. Handbook), Glushko, V.P., Ed., VINITI, 1965–1981, vol. 4, part 1, vol. 6, part 1.
Gurvich, L.V., Karachevtsev, G.V., Kondrat’ev, V.N., et al., Energii razryva khimicheskikh svyazei. Potentsialy ionizatsii i srodstvo k elektronu (Chemical Bond Energies. Ionization Potentials and Electron Affinities), Moscow: Nauka, 1974.
Karapet’yants, M.Kh. and Karapet’yants, M.L., Spravochnik. Osnovnye termodinamicheskie konstanty neorganicheskikh i organicheskikh veshchestv (Handbook. Key Thermodynamic Characteristics of Inorganic and Organic Compounds), Moscow: Khimiya, 1968.
NIST Chemistry WebBook, Linstrom, P.J. and Mallard, W.G., Eds., NIST Standard Reference Database Number 69, Gaithersburg (MD): National Institute of Standards and Technology, 2023. https://doi.org/10.18434/T4D303
Kireev, V.A., Metody prakticheskikh raschetov v termodinamike khimicheskikh reaktsii (Practical Calculation Methods for Thermodynamics of Chemical Reactions), Moscow: Khimiya, 1970.
Christe, K.O. and Naumann, D., Spectrochim. Acta, Part A, 1973, vol. 29, no. 12, p. 2017. https://doi.org/10.1016/0584-8539(73)80060-1
Szczęsny, R. and Szłyk, E., J. Therm. Anal. Calorim., 2013, vol. 111, no. 2, p. 1325.
Li, H., Zhao, B., Ding, R., et al., Crystal Growth Design, 2012, vol. 12, no. 8, p. 4170.
Chirakkara, S., Nanda, K.K., and Krupanidhi, S.B., Thin Solid Films, 2011, vol. 519, p. 3647.
Bernik, S., Kosir, M., and Guilmeau, E., Zast. Mater., 2016, vol. 57, no. 2, p. 318.
Gholami, M., Khodadadi, A.A., Anaraki Firooz, A., et al., Sensors Actuators B, 2015, vol. 212, p. 395.
Ahmad, M., Zhao, J., Iqbal, J., et al., J. Phys. D: Appl. Phys., 2009, vol. 42, p. 165406.
Mishra, S. and Daniele, S., Chem. Rev., 2015, vol. 115, no. 16, p. 8379.
Hichou, A.E., Bougrine, A., Bubendorff, J.L., et al., Semicond. Sci. Technol., 2002, vol. 17, no. 6, p. 607.
Gunasekaran, E., Ezhilan, M., Mani, G., et al., Semicond. Sci. Technol., 2018, vol. 33, no. 9, p. 095005.
Antony, A., Pramodini, S., Kityk, I.V., et al., Physica. E, 2017, vol. 94, p. 190.
Kadi, M.W., McKinney, D., Mohamed, R.M., et al., Ceramics Intern., 2016, vol. 42, no. 4, p. 4672.
Choi, Y.-J. and Park, H.-H., J. Mater. Chem., 2014, vol. 2, no. 1, p. 98.
Cosham, S.D., Kociok-Köhn, G., Johnson, A.L., et al., Eur. J. Inorg. Chem., 2015, vol. 2015, no. 26, p. 4362.
ACKNOWLEDGMENTS
The studies were carried out using the equipment of the Center for Collective Use of Physical Methods of Investigation at the Kurnakov Institute of General and Inorganic Chemistry (Russian Academy of Sciences).
Funding
This work was supported by the Russian Science Foundation, project no. 21-13-00086.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author of this work declares that they has no conflicts of interest.
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Malkerova, I.P., Kayumova, D.B., Belova, E.V. et al. Heterophase Synthesis of Silver Trifluoroacetate with Copper, Indium, and Zinc. Standard Enthalpy of Formation of Copper Trifluoroacetate. Russ J Coord Chem 49, 730–734 (2023). https://doi.org/10.1134/S1070328423600237
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328423600237