Abstract
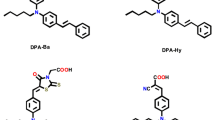
Dimethylphenyldihydroacridine dyes are an important type of organic molecule that are used in dye-sensitized solar cells. Two new organic dyes, namely (E)-3-(7-bromo-9,9-dimethyl-10-phenyl-9,10-dihydroacridin-2-yl)acrylic acid (DPAA) and (E)-3-(7-bromo-9,9-dimethyl-10-phenyl-9,10-dihydroacridin-2-yl)-2-cyanoacrylic acid (DPACA) were synthesized and developed as photosensitizers for DSSCs. The DPAA and DPACA dyes are characterized using Fourier Transform Infrared (FT-IR), nuclear magnetic resonance spectroscopy (NMR), and Ultraviolet-visible (UV–Vis) spectroscopy. Cyclic voltammetry (CV) and Density functional theory (DFT) calculations have been used to evaluate the energy level of dyes. The electronic excitations and charge transport properties are investigated using time-dependent density functional theory (TD-DFT) methods. The energy levels of Highest occupied molecular orbital (HOMO) and Lowest unoccupied molecular orbital (LUMO) molecular orbitals can be tuned by varying the π-conjugated units and the donating possibility of the donor part. The relationship between the structure of the dye and the photophysical, photovoltaic, and performance characteristics of Dye-sensitized solar cells (DSSCs) is investigated in depth. In addition, some quantitative parameters influencing the efficiency of power conversion, such as electron injection driving and light-harvesting efficiency have been calculated to identify the organic dyes for DSSCs applications.













Similar content being viewed by others
REFERENCES
L. L. Estrella, S. H. Lee, and D. H. Kim, Dyes Pigm. 165, 1 (2019). https://doi.org/10.1016/j.dyepig.2019.02.002
M. Megala, Beulah, and J. M. Rajkumar, J. Comput. Electron. 18, 1128 (2019). https://doi.org/10.1007/s10825-019-01398-0
K. Periyasamy, P. Sakthivel, P. Vennila, P. M. Anbarasan, G. Venkatesh, and Y. Sheena Mary, J. Photochem. Photobiol. A 413, 113269 (2021). https://doi.org/10.1016/j.jphotochem.2021.113269
O. Britel, A. Fitri, A. T. Benjelloun, A. Slimi, M. Benzakour, and M. Mcharfi, J. Photochem. Photobiol. A 429, 113902 (2022). https://doi.org/10.1016/j.jphotochem.2022.113902
K. Periyasamy P. Sakthivel, G. Venkatesh, P. M. Anbarasan, P. Vennila, Y. Sheena Mary, S. Kaya, and S. Erkan, J. Mol. Model. 28, 34 (2022). https://doi.org/10.1007/s00894-022-05026-w
S. A. H. Vuai, M. Salum Khalfan, and N. Surendra Babu, Heliyon 7, e08339 (2021). https://doi.org/10.1016/j.heliyon.2021.e08339
M. Megala, Beulah, and J. M. Rajkumar, J. Comput. Electron. 17, 1153 (2018). https://doi.org/10.1007/s10825-018-1195-8
H. Cheng, Y. Wu, J. Su, Z. Wang, R. Prasad Ghimire, M. Liang, Z. Sun, and S. Xue, Dyes Pigm. 149, 16 (2018). https://doi.org/10.1016/j.dyepig.2017.09.053
Zh.-B. Cai, Sh.-Sh. Liu, B. Li, Q.-J. Dong, Z.-L. Liu, M. Zheng, Sh.-L. Li, Y.-P. Tian, L.-J. Chen, and Q. Ye, Dyes Pigm. 165, 200 (2019). https://doi.org/10.1016/j.dyepig.2019.01.032
J.-M. Ji, H. Zhou, Y. K. Eom, Ch. H. Kim, and H. K. Kim, Adv. Energy Mater. 10, 2000124 (2020). https://doi.org/10.1002/aenm.202000124
T. Saravana Kumaran, A. Prakasam, P. M. Anbarasan, P. Vennila, G. Venkatesh, S. Parveen Banu, and Y. Sheena Mary, J. Comput. Biophys. Chem. 20, 465 (2021). https://doi.org/10.1142/S2737416521500253
Y. K. Eom, S. H. Kang, I. T. Choi, Y. Yoo, J. Kim, and H. K. Kim, J. Mater. Chem. A. 5, 2297 (2017). https://doi.org/10.1039/C6TA09836C
L. Li, Y. Wu, Q. Zhou, and Ch. He, J. Phys. Org. Chem. 25, 362 (2012). https://doi.org/10.1002/poc.1923
D. Joly, L. Pellejà, S. Narbey, F. Oswald, T. Meyer, Y. Kervella, P. Maldivi, J. N. Clifford, E. Palomares, and R. Demadrille, Energy Environ. Sci. 8, 2010 (2015). https://doi.org/10.1039/C5EE00444F
S. Sudhaker Reddy, W. Cho, V. Gopalan Sree, and S. H. Jin, Dyes Pigm. 134, 324 (2016). https://doi.org/10.1016/j.dyepig.2016.07.034
M. Hosseinnezhad, S. Moradian, K. Gharanjig, and F. Afshar Taromi, Adv. Perform. Mater. 29, 112 (2014). https://doi.org/10.1179/1753555713Y.0000000107
S. Fukuzumi, H. Kotani, Y. M. Lee, and W. Nam, J. Am. Chem. Soc. 130, 15134 (2008). https://doi.org/10.1021/ja804969k
R. Tarsang, V. Promarak, T. Sudyoadsuk, S. Namuangruk, N. Kungwan, and S. Jungsuttiwong, Chem. Phys. Chem. 15, 3809 (2014). https://doi.org/10.1002/cphc.201402458
L. L. Estrella, S. Hee Lee, and D. Hee Kim, Dyes Pigm. 165, 1 (2019). https://doi.org/10.1016/j.dyepig.2019.02.002
M. P. Balanay, C. M. Enopia, S. H. Lee, and D. H. Kim, Spectrochim. Acta, Part A 104, 382 (2015). https://doi.org/10.1016/j.saa.2015.01.002
L. L. Estrella, M. P. Balanay, and D. H. Kim, J. Phys. Chem. A 120, 5917 (2016). https://doi.org/10.1021/acs.jpca.6b03271
S. H. Kim, H. W. Kim, C. Sakong, J. Namgoong, S. W. Park, M. J. Ko, C. H. Lee, W. I. Lee, and J. P. Kim, Org. Lett. 13, 5784 (2011). https://doi.org/10.1021/ol2023517
B. H. Kim and H. S. Freeman, J. Mater. Chem. 22, 20403(2012). https://doi.org/10.1039/C2JM33228K
J. Li, Z. Zhuang, X. Zhu, Z. Zhao, and B. Zh. Tang, J. Soc. Inf. Disp. 2, 139 (2020). https://doi.org/10.1080/15980316.2020.1784805
D. Cheng, D. Xu, Y. Wang, H. Zhou, Z. Zhou, X. Liu, A. Han, and C. Zhang, Dyes Pigm. 173, 107937 (2020). https://doi.org/10.1016/j.dyepig.2019.107937
T. Saravanakumaran, A. Prakasam, G. Venkatesh, C. Kamal, Y. Sheena Mary, S. Parveen Banu, P. Vennila, and Y. Shyma Mary, Zeitschr. Phys. Chem. 235, 1355 (2021). https://doi.org/10.1515/zpch-2020-1732
X. Xu, Z. Cao, and Q. Zhang, J. Chem. Phys. 122, 194305 (2005). https://doi.org/10.1063/1.1895673
Y. Ait Aicha, S. M. Bouzzine, T. Zair, M. Bouachrine, M. Hamidi, Z. Mohyieddine Fahim, G. Salgado Moran, L. Mendoza Huizar, L. Alvarado Soto, and R. Ramirez Tagle, J. Theor. Comput. Chem. 15, 165002 (2016). https://doi.org/10.1142/S0219633616500231
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, et al., Gaussian 16, Revision A.03 (Gaussian Inc., Wallingford, CT, 2016).
A. El Alamy, M. Bourass, A. Amine, and M. Hamidi, Int. J. Mod. Sci. 3, 75 (2017). https://doi.org/10.1016/j.kijoms.2017.03.002
W. S. Aroon, S. Laopha, P. Chaiamornnugool, S. Tontapha, S. Saekow, and V. Amornkitbamrung, J. Mol. Model. 19, 1407 (2013). https://doi.org/10.1007/s00894-012-1692-9
X. Yang, J. Walpita, D. Zhou, H. Ling Luk, Sh. Vyas, R. S. Khnayzer, S. C. Tiwari, K. Diri, Ch. M. Hadad, F. N. Castellano, A. I. Krylova, and K. D. Glusac, J. Phys. Chem. B 117, 15290 (2013). https://doi.org/10.1021/jp401770e
D. S. Patil, K. C. Avhad, and N. Sekar, Comput. Theor. Chem. 1138, 75 (2018). https://doi.org/10.1016/j.comptc.2018.06.006
S. A. Elroby and A. Jedidi, Struct. Chem. 31, 1125 (2020). https://doi.org/10.1007/s11224-020-01489-w
D. Devadiga, M. Selvakumar, P. Shetty, M. G. Mahesha, D. Devadiga, T. N. Ahipa, and S. Senthil Kumar, J. Solid State Electrochem. 25, 1461 (2021). https://doi.org/10.1007/s10008-021-04920-2
M. S. Abusaif, M. Fathy, M. A. Abu-Saied, A. A. Elhenawy, A. B. Kashyout, M. R. Selim, and Y. A. Ammar, J. Mol. Struct. 1225, 129297 (2021). https://doi.org/10.1016/j.molstruc.2020.129297
S. Mandal, G. R. Kandregula, and V. Naga Baji Tokala, J. Photochem. Photobiol. A 401, 112745 (2020). https://doi.org/10.1016/j.jphotochem.2020.112745
Y. Sheng Yen, J. Ling Hsu, Y. Tse Cheng, Y. Chan Hsu, and J. T. Lin, Mol. Cryst. Liq. Cryst. 703, 32 (2020). https://doi.org/10.1080/15421406.2020.1743940
S. F. Abdulhussein, S. M. Abdalhadi, and H. D. Hanoon, Egypt. J. Chem. 65, 211 (2022). https://doi.org/10.21608/ejchem.2022.115059.5221
R. M. El-Shishtawy, A. M. Asiri, S. G. Aziz, and A. K. Elroby Shaaban, J. Mol. Model. 20, 2241 (2014). https://doi.org/10.1007/s00894-014-2241-5
S. Gauthier, F. Robin-Le Guen, L. Wojcik, N. L Poul, A. Planchat, Y. Pellegrin, P. Guevara Level, N. Szuwarski, M. Boujtita, D. Jacquemin, and F. Odobel, Solar Energy 205, 310 (2020). https://doi.org/10.1016/j.solener.2020.05.036
T. Saravana Kumaran, A. Prakasam, P. Vennila, S. Parveen Banu, and G. Venkatesh, Asian J. Chem. 33, 1541 (2021). https://doi.org/10.14233/ajchem.2021.23197
R. Kesavan, I. M. Abdellah, S. P. Singh, A. El-Shafei, and A. V. Adhikari, Phys. Chem. Chem. Phys. 21, 10603 (2019). https://doi.org/10.1039/C9CP01032G
P. Vennila, M. Govindaraju, G. Venkatesh, C. Kamal, Y. Sheena Mary, C. Yohannan Panicker, S. Kaya, St. Armakovi, and S. J. Armakovi, J. Mol. Struct. 1151, 245 (2018). https://doi.org/10.1016/j.molstruc.2017.09.049
A. K. Mishra and S. P. Tewari, SN Appl. Sci. 2, 1021 (2020). https://doi.org/10.1007/s42452-020-2842-9
D. A. Kleinman, Phys. Rev. 26, 1977 (1962). https://doi.org/10.1103/PhysRev.126.1977
M. Hachi, A. Slimi, A. Fitri, A. Touimi Benjelloun, S. El Khattabi, M. Benzakour, M. Mcharfi, M. Khenfouch, I. Zorkani, and M. Bouachrine, J. Photochem. Photobiol. A 407, 113048 (2021). https://doi.org/10.1016/j.jphotochem.2020.113048
M. R. Elmorsy, E. Abdel-Latif, S. A. Badawy, and A. A. Fadda, J. Photochem. Photobiol., A 389, 112239 (2020). https://doi.org/10.1016/j.jphotochem.2019.112239
M. M. Jadhav, J. V. Vaghasiya, D. Patil, S. S. Soni, and N. Sekar, J. Photochem. Photobiol., A 377, 119 (2019). https://doi.org/10.1016/j.jphotochem.2019.03.043
P. Vennila, G. Venkatesh, Y. Sixto-López, C. Kamal, S. Kaya, G. Serdaroğlu, and B. Landeros-Rivera, J. Mol. Struct. 1246, 131164 (2021). https://doi.org/10.1016/j.molstruc.2021.131164
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Supplementary Information
Rights and permissions
About this article
Cite this article
Saravana Kumaran, T., Prakasam, A., Venkatesh, G. et al. Design and Synthesis of Phenylacridine-Based on Organic Dyes and Its Applications in Dye-Sensitized Solar Cells. Russ. J. Phys. Chem. 97, 2607–2623 (2023). https://doi.org/10.1134/S0036024423110298
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423110298