Abstract
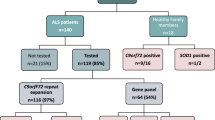
An intronic bi-allelic pentanucleotide repeat expansion mutation, (AAGGG)400–2000, at AAAAG repeat locus in RFC1 gene, is known as underlying genetic cause in cases with cerebellar ataxia, neuropathy, and vestibular areflexia syndrome (CANVAS) and late-onset sporadic ataxia. Biallelic positive cases carry a common recessive risk haplotype, “AAGA,” spanning RFC1 gene. In this study, our aim is to find prevalence of bi-allelic (AAGGG)exp in Indian ataxia and other neurological disorders and investigate the complexity of RFC1 repeat locus and its potential association with neurodegenerative diseases in Indian population-based cohorts. We carried out repeat number and repeat type estimation using flanking PCR and repeat primed PCR (AAAAG/AAAGG/AAGGG) in four Indian disease cohorts and healthy controls. Haplotype assessment of suspected cases was done by genotyping and confirmed by Sanger sequencing. Blood samples and consent of all the cases and detailed clinical details of positive cases were collected in collaboration with A.I.I.M.S. Furthermore, comprehension of RFC1 repeat locus and risk haplotype analysis in Indian background was performed on the NGS data of Indian healthy controls by ExpansionHunter, ExpansionHunter Denovo, and PHASE analysis, respectively. Genetic screening of RFC1-TNR locus in 1998 uncharacterized cases (SCA12: 87; uncharacterized ataxia: 1818, CMT: 93) and 564 heterogenous controls showed that the frequency of subjects with bi-allelic (AAGGG)exp are 1.15%, < 0.05%, 2.15%, and 0% respectively. Two RFC1 positive sporadic late-onset ataxia cases, one bi-allelic (AAGGG)exp and another, (AAAGG)~700/(AAGGG)exp, had recessive risk haplotype and CANVAS symptoms. Long normal alleles, 15–27, are significantly rare in ataxia cohort. In IndiGen control population (IndiGen; N = 1029), long normal repeat range, 15–27, is significantly associated with A3G3 and some rare repeat motifs, AGAGG, AACGG, AAGAG, and AAGGC. Risk-associated “AAGA” haplotype of the original pathogenic expansion of A2G3 was found associated with the A3G3 representing alleles in background population. Apart from bi-allelic (AAGGG)exp, we report cases with a new pathogenic expansion of (AAAGG)exp/(AAGGG)exp in RFC1 and recessive risk haplotype. We found different repeat motifs at RFC1 TNR locus, like AAAAG, AAAGG, AAAGGG, AAAAGG, AAGAG, AACGG, AAGGC, AGAGG, and AAGGG, in Indian background population except ACAGG and (AAAGG)n/(AAGGG)n. Our findings will help in further understanding the role of long normal repeat size and different repeat motifs, specifically AAAGG, AAAGGG, and other rare repeat motifs, at the RFC1 locus.
Graphical Abstract



Similar content being viewed by others
Abbreviations
- CANVAS:
-
Cerebellar ataxia, neuropathy, and vestibular areflexia syndrome
- f-PCR:
-
Flanking PCR
- RP-PCR:
-
Repeat primed PCR
- LR-PCR:
-
Long range PCR
- EHDn:
-
ExpansionHunter Denovo
- IndiGen:
-
Indian genomes
- ADL:
-
Activities of daily living
- ROS:
-
Reactive oxygen species
- STR:
-
Short tandem repeats
- TNR:
-
Tandem nucleotide repeats
References
Faruq M, Scaria V, Singh I, Tyagi S, Srivastava AK, Mukerji M (2009) SCA-LSVD: a repeat-oriented locus-specific variation database for genotype to phenotype correlations in spinocerebellar ataxias. Hum Mutat 30(7):1037–1042. https://doi.org/10.1002/humu.21006
Shakya S, Kumari R, Suroliya V et al (2019) Whole exome and targeted gene sequencing to detect pathogenic recessive variants in early onset cerebellar ataxia. Clin Genet 96(6):566–574. https://doi.org/10.1111/cge.13625
Uppili B, Sharma P, Ahmad I, Sahni S, Asokachandran V, Nagaraja AB, Srivastava AK, Faruq M (2023) Sequencing through hyperexpanded Friedreich's ataxia-GAA repeats by nanopore technology: implications in genotype-phenotype correlation. Brain Commun 5(2):fcad020. https://doi.org/10.1093/braincomms/fcad020
Sharma P, Sonakar AK, Tyagi N, Suroliya V, Kumar M, Kutum R, Asokchandran V, Ambawat S, Shamim U, Anand A, Ahmad I, Shakya S, Uppili B, Mathur A, Parveen S, Jain S, Singh J, Seth M, Zahra S et al (2022) Genetics of ataxias in Indian population: a collative insight from a common genetic screening tool. Adv Genet 3(2):2100078. https://doi.org/10.1002/GGN2.202100078
Bahl S, Virdi K, Mittal U, Sachdeva MP, Kalla AK, Holmes SE, O'Hearn E, Margolis RL, Jain S, Srivastava AK, Mukerji M (2005) Evidence of a common founder for SCA12 in the Indian population. Ann Hum Genet 69(Pt 5):528–534. https://doi.org/10.1046/j.1529-8817.2005.00173.x
Faruq M, Srivastava AK, Suroliya V, Kumar D, Garg A, Shukla G, Behari M (2014) Identification of FXTAS presenting with SCA 12 like phenotype in India. Parkinsonism Relat Disord 20(10):1089–1093. https://doi.org/10.1016/j.parkreldis.2014.07.001
Kumar D, Hussain A, Srivastava AK, Mukerji M, Mukherjee O, Faruq M (2018) Generation of three spinocerebellar ataxia type-12 patients derived induced pluripotent stem cell lines (IGIBi002-A, IGIBi003-A and IGIBi004-A). Stem Cell Res 31:216–221. https://doi.org/10.1016/j.scr.2018.08.008
Cortese A, Simone R, Sullivan R, Vandrovcova J, Tariq H, Yau WY, Humphrey J, Jaunmuktane Z, Sivakumar P, Polke J, Ilyas M (2019) Biallelic expansion of an intronic repeat in RFC1 is a common cause of late-onset ataxia. Nat Genet 51(4):649–658. https://doi.org/10.1038/s41588-019-0372-4
Scriba CK, Beecroft SJ, Clayton JS, Cortese A, Sullivan R, Yau WY, Dominik N, Rodrigues M, Walker E, Dyer Z, Wu TY (2020) A novel RFC1 repeat motif (ACAGG) in two Asia-Pacific CANVAS families. Brain 143(10):2904–2910. https://doi.org/10.1093/BRAIN/AWAA263
Akçimen F, Ross JP, Bourassa CV, Liao C, Rochefort D, Gama MTD, Dicaire MJ, Barsottini OG, Brais B, Pedroso JL, Dion PA (2019) Investigation of the RFC1 repeat expansion in a Canadian and a Brazilian ataxia cohort: identification of novel conformations. Front Genet 10:1219. https://doi.org/10.3389/fgene.2019.01219
Dupré M, Hermann R, Froment Tilikete C (2021) Update on cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome (CANVAS). The Cerebellum:1–14. https://doi.org/10.1007/S12311-020-01192-W
Dominik N, Galassi Deforie V, Cortese A, Houlden H (2021) CANVAS: a late onset ataxia due to biallelic intronic AAGGG expansions. J Neurol 268:1119–1126. https://doi.org/10.1007/s00415-020-10183-0
Beecroft SJ, Cortese A, Sullivan R et al (2020) A Māori specific RFC1 pathogenic repeat configuration in CANVAS, likely due to a founder allele. Brain 143(9):2673–2680. https://doi.org/10.1093/brain/awaa203
Chintalaphani SR, Pineda SS, Deveson IW, Kumar KR (2021) An update on the neurological short tandem repeat expansion disorders and the emergence of long-read sequencing diagnostics. Acta Neuropathol Commun 9(1). https://doi.org/10.1186/s40478-021-01201-x
Cortese A, Simone R, Sullivan R, Vandrovcova J, Tariq H, Yau WY. ..., Houlden H (2019) Biallelic expansion of an intronic repeat in RFC. 51(4):649–658. https://doi.org/10.1038/s41588-019-0372-4
Rafehi H, Szmulewicz DJ, Bennett MF et al (2019) Bioinformatics-Based Identification of Expanded Repeats: A Non-reference Intronic Pentamer Expansion in RFC1 causes CANVAS. Am J Hum Genet 105(1):151–165. https://doi.org/10.1016/j.ajhg.2019.05.016
Davies K, Szmulewicz DJ, Corben LA, Delatycki M, Lockhart PJ (2022) RFC1-related disease molecular and clinical insights. Neurol Genet 8(5):5. https://doi.org/10.1212/NXG.0000000000200016
Majka J, Burgers PMJ (2004) The PCNA-RFC families of DNA clamps and clamp loaders. Prog Nucleic Acid Res Mol Biol 78:227–260. https://doi.org/10.1016/S0079-6603(04)78006-X
Ogi T, Limsirichaikul S, Overmeer RM et al (2010) Three DNA polymerases, recruited by different mechanisms, carry out NER repair synthesis in human cells. Mol Cell 37(5):714–727. https://doi.org/10.1016/j.molcel.2010.02.009
Tagliapietra M, Cardellini D, Ferrarini M et al (2021) RFC1 AAGGG repeat expansion masquerading as chronic idiopathic axonal polyneuropathy. J Neurol 2021(1):1–11. https://doi.org/10.1007/S00415-021-10552-3
Boesch SM, Nance MA (2020) Intronic pentanucleotide expansion in the replication factor 1 gene (RFC1) is a major cause of adult-onset ataxia. Neurol Genet 6(3). https://doi.org/10.1212/NXG.0000000000000436
Kytövuori L, Sipilä J, Doi H et al (2022) Biallelic expansion in RFC1 as a rare cause of Parkinson’s disease. NPJ Parkinsons Dis 8(1):6. https://doi.org/10.1038/s41531-021-00275-7
Fan Y, Zhang S, Yang J et al (2020) No biallelic intronic AAGGG repeat expansion in RFC1 was found in patients with late-onset ataxia and MSA. Parkinsonism Relat Disord 73:1–2. https://doi.org/10.1016/j.parkreldis.2020.02.017
Kumar KR, Cortese A, Tomlinson SE et al (2020) RFC1 expansions can mimic hereditary sensory neuropathy with cough and Sjögren syndrome. Brain 143(10):e82–e82. https://doi.org/10.1093/brain/awaa244
Sullivan R, Yau WY, Chelban V et al (2021) RFC1-related ataxia is a mimic of early multiple system atrophy. J Neurol Neurosurg Psychiatry. 92(4):444–446. https://doi.org/10.1136/JNNP-2020-325092
Currò R, Salvalaggio A, Tozza S et al (2021) RFC1 expansions are a common cause of idiopathic sensory neuropathy. Brain 144(5):1542–1550. https://doi.org/10.1093/BRAIN/AWAB072
Wan L, Chen Z, Wan N, Liu M, Xue J, Chen H et al (2020) Biallelic intronic AAGGG expansion of RFC1 is related to multiple system atrophy. Ann Neurol 88(6):1132–1143. https://doi.org/10.1002/ana.25902
Van Daele SH, Vermeer S, Van Eesbeeck A et al (2020) Diagnostic yield of testing for RFC1 repeat expansions in patients with unexplained adult-onset cerebellar ataxia. J Neurol Neurosurg Psychiatry 91(11):1233–1234. https://doi.org/10.1136/jnnp-2020-323998
Reilly MM (2021) RFC1 CANVAS: the expanding phenotype. J Neurol Neurosurg Psychiatry 92(4):345–345. https://doi.org/10.1136/JNNP-2020-325504
Traschütz A, Cortese A, Reich S et al (2021) Natural history, phenotypic spectrum, and discriminative features of multisystemic RFC1 disease. Neurology 96(9). https://doi.org/10.1212/WNL.0000000000011528
Beijer D, Dohrn MF, De Winter J, Fazal S, Cortese A, Stojkovic T et al (2022) RFC1 repeat expansions: a recurrent cause of sensory and autonomic neuropathy with cough and ataxia. Eur J Neurol 29(7):2156–2161. https://doi.org/10.1111/ene.15310
Currò R, Salvalaggio A, Tozza S, Gemelli C, Dominik N, Galassi Deforie V et al (2021) RFC1 expansions are a common cause of idiopathic sensory neuropathy. Brain 144(5):1542–1550. https://doi.org/10.1093/BRAIN/AWAB072
Gazulla J, Pablo-Zaro MJ, Fraile-Rodrigo J, Larrodé P (2016) Sensory neuronopathy in CANVAS: cerebellar ataxia, neuropathy, and vestibular areflexia. Can J Neurol Sci 43(4):604–605. https://doi.org/10.1017/cjn.2015.396
Cortese A, Tozza S, Yau WY et al (2020) Cerebellar ataxia, neuropathy, vestibular areflexia syndrome due to RFC1 repeat expansion. Brain 143(2):489–490. https://doi.org/10.1093/brain/awz418
Yacovino DA, Zanotti E (2019) Is cerebellar ataxia, neuropathy, and vestibular areflexia syndrome (Canvas) a vestibular ganglionopathy? J Int Adv Otol 15(2):304–308. https://doi.org/10.5152/iao.2019.7068
Abramzon Y, Dewan R, Cortese A,et al (2021) Investigating RFC1 expansions in sporadic amyotrophic lateral sclerosis. J Neurol Sci 430. https://doi.org/10.1016/j.jns.2021.118061
Huin V, Coarelli G, Guemy C et al (2022) Motor neuron pathology in CANVAS due to RFC1 expansions. Brain 145(6):2121–2132. https://doi.org/10.1093/brain/awab449
Holmes SE, O’Hearn E, Ross CA, Margolis RL (2001) SCA12: an unusual mutation leads to an unusual spinocerebellar ataxia. Brain Res Bull 56(3–4):397–403. https://doi.org/10.1016/S0361-9230(01)00596-2
Holmes SE, O'Hearn EE, McInnis MG, Gorelick-Feldman DA, Kleiderlein JJ, Callahan C et al (1999) Expansion of a novel CAG trinucleotide repeat in the 5′ region of PPP2R2B is associated with SCA12. Nat Genet 23(4):391–392. https://doi.org/10.1038/70493
Srivastava AK, Takkar A, Garg A, Faruq M (2017) Clinical behaviour of spinocerebellar ataxia type 12 and intermediate length abnormal CAG repeats in PPP2R2B. Brain 140(1):27–36. https://doi.org/10.1093/brain/aww269
Bird TD (2022) Charcot-Marie-Tooth hereditary neuropathy overview. https://www.ncbi.nlm.nih.gov/books/NBK1358/
Jain A, Bhoyar RC, Pandhare K, Mishra A, Sharma D, Imran M et al (2021) IndiGenomes: a comprehensive resource of genetic variants from over 1000 Indian genomes. Nucleic Acids Res 49(D1):D1225–D1232. https://doi.org/10.1093/NAR/GKAA923
Dolzhenko E, Bennett MF, Richmond PA, Trost B, Chen S, van Vugt JJ et al (2020) ExpansionHunter Denovo: a computational method for locating known and novel repeat expansions in short-read sequencing data. Genome Biol 21:1–14. https://doi.org/10.1186/s13059-020-02017-z
Dolzhenko E, Deshpande V, Schlesinger F, Krusche P, Petrovski R, Chen S et al (2019) ExpansionHunter: a sequence-graph-based tool to analyze variation in short tandem repeat regions. Bioinformatics 35(22):4754–4756. https://doi.org/10.1093/bioinformatics/btz431
Stephens M, Donnelly P (2003) A comparison of Bayesian methods for haplotype reconstruction from population genotype data. Am J Human Genet 73(5):1162–1169. https://doi.org/10.1086/379378
Gardiner SL, Boogaard MW, Trompet S, de Mutsert R, Rosendaal FR, Gussekloo J et al (2019) Prevalence of carriers of intermediate and pathological polyglutamine disease–associated alleles among large population-based cohorts. JAMA Neurol 76(6):650–656. https://doi.org/10.1001/jamaneurol.2019.0423
Laffita-Mesa JM, Velázquez-Pérez LC, Santos Falcón N et al (2012) Unexpanded and intermediate CAG polymorphisms at the SCA2 locus (ATXN2) in the Cuban population: evidence about the origin of expanded SCA2 alleles. Eur J Hum Genet 20(1):41–49. https://doi.org/10.1038/ejhg.2011.154
GhahremaniNezhad H, Franklin JP, Alix JJP et al (2021) Simultaneous ALS and SCA2 associated with an intermediate-length ATXN2 CAG-repeat expansion. Amyotroph Lateral Scler Frontotemporal Degener 22(7–8):579–582. https://doi.org/10.1080/21678421.2020.1853172
Alonso I, Jardim LB, Artigalas O et al (2006) Reduced penetrance of intermediate size alleles in spinocerebellar ataxia type 10. Neurology 66(10):1602–1604. https://doi.org/10.1212/01.wnl.0000216266.30177.bb
Ronco R, Perini C, Currò R et al (2023) Truncating Variants in RFC1 in cerebellar ataxia, neuropathy, and vestibular areflexia syndrome. Neurology 100(5):E543–E554. https://doi.org/10.1212/WNL.0000000000201486/VIDEO-1
King KA, Wegner DJ, Bucelli RC, Shapiro J, Paul AJ, Dickson PI, Wambach JA (2022) Whole-genome and long-read sequencing identify a novel mechanism in RFC1 resulting in CANVAS syndrome. Neurol Genet 8(6). https://doi.org/10.1212/NXG.0000000000200036
Acknowledgements
We acknowledge Prof. Mitali Mukerji’s initial direction towards this work. We sincerely appreciate everyone who has provided constant support during this study, including lab assistants Subhash Gurjar, Usha Rawat, and Suman Mudila. We are thankful for the participation of the patients and their families.
Funding
Funding support from MLP1802, OLP1120 CSIR funded project. M.F. thanks C.S.I.R.-I.G.I.B. for research grant. N.T. is supported by Union Grant Commission (U.G.C.). P.S. has been supported by ICMR-SRF. Additionally, S.S.’s Department of Biotechnology (DBT-JRF) fellowship is acknowledged. V.A.’s Indian Council of Medical Research (ICMR-SRF) fellowship is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tyagi, N., Uppili, B., Sharma, P. et al. Investigation of RFC1 tandem nucleotide repeat locus in diverse neurodegenerative outcomes in an Indian cohort. Neurogenetics 25, 13–25 (2024). https://doi.org/10.1007/s10048-023-00736-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-023-00736-6