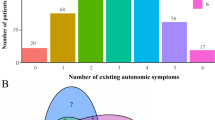
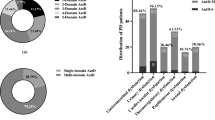
Abstract
Autonomic dysfunction (AutD) is common and debilitating in Parkinson’s disease (PD). Predictors of AutD are unclear, and data are limited on the biological relevance of AutD in PD. Here, we evaluated the baseline prevalence and 2-year longitudinal assessment of AutD in patients with de novo PD compared with healthy controls (HC). Moreover, we also assessed various variables that could predict longitudinal changes in AutD in early PD. Parkinson’s Progression Markers Initiative (PPMI) was utilized to evaluate untreated PD participants at baseline and HC. Autonomic function was assessed using the 25-item Scale for Outcomes in Parkinson’s Disease-Autonomic (SCOPA-AUT) score at baseline and 2 years. Clinical and biological variables were measured for their correlations with AuD for up to 2 years. Two hundred and ninety PD subjects and 170 HC were enrolled and followed for 2 years. SCOPA-AUT mean (SD) scores increased from baseline 8.49 ± 5.23 to 10.12 ± 5.77 at year 2 in PD subjects (p < 0.001) versus from 4.98 ± 3.34 to 5.03 ± 374 in HC (p = 0.496), with a significant difference between the groups (p < 0.001). Among them, 242 PD participants and 151 HC completed the SCOPA-AUT assessment, including sexual function. In the multivariate analysis, a higher baseline SCOPA-AUT score was associated with higher baseline MDS-UPDRS Part I scores (p < 0.001). Moreover, a longitudinal increase in autonomic function severity was associated with the white race (p = 0.010) at baseline. In contrast, there was no association with the CSF biomarkers. MDS-UPDRS Part I score may predict AuD in patients with early PD, which is correlated with nonmotor symptoms and race.
Similar content being viewed by others
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Aliev G et al (2003) Role of vascular hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of Azheimer disease. Neurotox Res 5(7):491–504
Arnao V et al (2015) In patient’s with Parkinson disease, autonomic symptoms are frequent and associated with other non-motor symptoms. Clin Auton Res 25(5):301–307
Berg A et al (2022) Autonomic dysfunction in Parkinson’s disease: results from the Faroese Parkinson’s disease cohort. Neurosci Lett 785:136789
Bostantjopoulou S et al (2016) Self-reported autonomic symptoms in Parkinson’s disease: properties of the SCOPA-AUT scale. Hippokratia 20(2):115–120
Braak H et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Brockmann K et al (2011) GBA-associated PD presents with nonmotor characteristics. Neurology 77(3):276–280
Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8(5):464–474
Coon EA, Cutsforth-Gregory JK, Benarroch EE (2018) Neuropathology of autonomic dysfunction in synucleinopathies. Mov Disord 33(3):349–358
Cui J et al (2021) Activities of daily living as a longitudinal moderator of the effect of autonomic dysfunction on anxiety and depression of Parkinson’s patients. Brain Behav 11(8):e2297
de la Torre JC (2000) Critically attained threshold of cerebral hypoperfusion: the CATCH hypothesis of Alzheimer’s pathogenesis. Neurobiol Aging 21(2):331–342
De Pablo-Fernandez E et al (2017) Association of autonomic dysfunction with disease progression and survival in parkinson disease. JAMA Neurol 74(8):970–976
Forjaz MJ et al (2010) Assessing autonomic symptoms of Parkinson’s disease with the SCOPA-AUT: a new perspective from Rasch analysis. Eur J Neurol 17(2):273–279
Gray WK, Wood BH, Walker RW (2009) Do autonomic function tests in people with Parkinson’s disease predict survival rates at 7 years follow-up? Mov Disord 24(16):2432–2434
Hinkle JT et al (2018) Dopamine transporter availability reflects gastrointestinal dysautonomia in early Parkinson disease. Parkinsonism Relat Disord 55:8–14
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376
Kendall PC et al (1976) The state-trait anxiety inventory: a systematic evaluation. J Consult Clin Psychol 44(3):406–412
Khoo TK et al (2013) The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 80(3):276–281
Kim JY et al (2017) Validation of the Korean version of the scale for outcomes in Parkinson’s disease-autonomic. J Mov Disord 10(1):29–34
Ledda C et al (2022) Burden of caregiving for cardiovascular dysautonomia in Parkinson’s disease. Clin Auton Res 32(6):455–461
Longardner K et al (2022) Differential impact of individual autonomic domains on clinical outcomes in Parkinson’s disease. J Neurol 269(10):5510–5520
Marek K et al (2011) The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 95(4):629–35
Merola A et al (2016) Orthostatic hypotension in Parkinson’s disease: does it matter if asymptomatic? Parkinsonism Relat Disord 33:65–71
Merola A et al (2018) Autonomic dysfunction in Parkinson’s disease: a prospective cohort study. Mov Disord 33(3):391–397
Nasreddine ZS et al (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699
Pazo JH, Belforte JE (2002) Basal ganglia and functions of the autonomic nervous system. Cell Mol Neurobiol 22(5–6):645–654
Petrucci S et al (2020) GBA-related Parkinson’s disease: dissection of genotype-phenotype correlates in a large Italian cohort. Mov Disord 35(11):2106–2111
Pilipovich AA et al (2022) Gastrointestinal dysfunction impact on life quality in a cohort of russian patients with Parkinson’s disease I-III H&Y stage. Parkinsons Dis 2022:1571801
Pilotto A et al (2019) Orthostatic hypotension and REM sleep behaviour disorder: impact on clinical outcomes in α-synucleinopathies. J Neurol Neurosurg Psychiatry 90(11):1257–1263
Pilotto A et al (2021) Association of orthostatic hypotension with cerebral atrophy in patients with Lewy body disorders. Neurology 97(8):e814–e824
Sklerov M et al (2020) Longitudinal change in autonomic symptoms predicts activities of daily living and depression in Parkinson’s disease. Clin Auton Res 30(3):223–230
Sklerov M et al (2022) Autonomic and depression symptoms in Parkinson’s disease: clinical evidence for overlapping physiology. J Parkinsons Dis 12(3):1059–1067
Stanković I et al (2019) Longitudinal assessment of autonomic dysfunction in early Parkinson’s disease. Parkinsonism Relat Disord 66:74–79
van Deursen DN et al (2020) Autonomic failure in Parkinson’s disease is associated with striatal dopamine deficiencies. J Neurol 267(7):1922–1930
Vichayanrat E et al (2021) Lower urinary tract dysfunction in Parkinsonian syndromes. Neurol Sci 42(10):4045–4054
Visser M et al (2004) Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov Disord 19(11):1306–1312
Winge K et al (2005) Relationship between nigrostriatal dopaminergic degeneration, urinary symptoms, and bladder control in Parkinson’s disease. Eur J Neurol 12(11):842–850
Yeh TL et al (2006) Correlation between striatal dopamine D2/D3 receptor binding and cardiovascular activity in healthy subjects. Am J Hypertens 19(9):964–969
Yu Z, Li Y, The Parkinson’s Progression Markers (2021) Association of autonomic symptoms with cerebrospinal fluid biomarkers in Parkinson disease and scans without evidence of dopaminergic deficit. Medicine (Baltimore). 100(7):e24837
Zhou Z et al (2021) Characteristics of autonomic dysfunction in parkinson’s disease: a large chinese multicenter cohort study. Front Aging Neurosci 13:761044
Funding
This study received no funding support.
Author information
Authors and Affiliations
Contributions
MY: conceived and designed the experiments. LY and HG: contributed significantly to the experiments, arranging data and performing data analyses. LY: wrote the draft manuscript. MY revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval and consent to participate
All patients provided their written, voluntarily informed consent. All procedures were carried out in accordance with the guidelines outlined in the Helsinki Declaration, and the Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University approved this study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, L., Gao, H. & Ye, M. Baseline prevalence and longitudinal assessment of autonomic dysfunction in early Parkinson’s disease. J Neural Transm 131, 127–139 (2024). https://doi.org/10.1007/s00702-023-02711-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-023-02711-9