Abstract
Purpose
To analyze interventions implemented at the time of colorectal cancer (CRC) screening, or among individuals who have previously undergone investigation for CRC, focused on reducing CRC risk through promotion of lifestyle behavior change. Additionally, this review evaluated to what extent such interventions apply behavior change techniques (BCTs) to achieve their objectives.
Methods
Five databases were systematically searched to identify randomized control trials seeking to reduce CRC risk through behavior change. Outcomes were changes in health-related lifestyle behaviors associated with CRC risk, including changes in dietary habits, body mass index, smoking behaviors, alcohol consumption, and physical activity. Standardized mean differences (SMDs) with 95% confidence intervals (CIs) were pooled using random effects models. BCT’s were coded from a published taxonomy of 93 techniques.
Results
Ten RCT’s met the inclusion criteria. Greater increase in fruit/vegetable consumption in the intervention group were observed with respect to the control (SMD 0.13, 95% CI 0.08 to 0.18; p < 0.001). Across fiber, alcohol, fat, red meat, and multivitamin consumption, and smoking behaviors, similar positive outcomes were observed (SMD 0.09–0.57 for all, p < 0.01). However, among physical activity and body mass index, no difference between the intervention groups compared with controls were observed. A median of 7.5 BCTs were applied across included interventions.
Conclusion
While magnitude of the observed effect sizes varied, they correspond to potentially important changes in lifestyle behaviors when considered on a population scale. Future interventions should identify avenues to maximize long-term engagement to promote sustained lifestyle behavior change.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the 3rd most common cancer globally and the 4th most common cancer in the United Kingdom, with approximately 100 newly diagnosed cases each day in the UK [1]. Current research attributes approximately 40% of CRC risk to body mass index and lifestyle factors such as physical activity, diet, smoking, and alcohol consumption [1]. As such, the risk of colorectal cancer can be reduced by modifying exposure to such lifestyle risk factors through reducing physical inactivity, being overweight or obese, and consumption of an unhealthy diet [2,3,4].
To reduce CRC incidence and mortality, many countries have established national colorectal cancer screening programs [5]. The English colorectal cancer screening program, started in 2006, has been associated with a 15% reduction in colorectal cancer mortality [6]. A growing body of literature describes colorectal cancer screening as a “teachable moment” to facilitate lifestyle-related behavior change that may reduce future CRC risk [7]. Managing these risk factors has also been shown to reduce the risk of developing other chronic conditions, including cardiovascular disease and type II diabetes mellitus.
A number of studies have delivered individual-level interventions at the time of colorectal cancer screening focused on reducing CRC risk via improving adherence to several lifestyle behaviors, including physical activity, diet, smoking, and alcohol consumption. These studies have shown different degrees of success in changing behavior [8,9,10]. A recent systematic review by Orange et al. synthesized the evidence on physical activity and dietary interventions among individuals attending colorectal and breast cancer screening [11]. However, that review does not address several other lifestyle behaviors associated with CRC risk, and excludes interventions delivered outside of the context of CRC screening that could feasibly be delivered within. Additionally, that review does not assess the application of behavior change techniques (BCTs) within existing interventions aimed at promoting positive changes of these specific cancer-preventive lifestyle behaviors. This is especially important given the utility of BCTs in identifying specific components of interventions that may be associated with behavior change, and to characterize behavior change focused interventions in general.
The goal of this review is to systematically analyze interventions implemented at the time of CRC screening, or among individuals who have previously undergone investigation for CRC, that are focused on reducing CRC risk through promotion of lifestyle behavior change. An additional goal of this review is to evaluate to what extent such interventions apply BCTs to achieve their objectives.
Methods
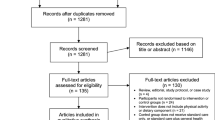
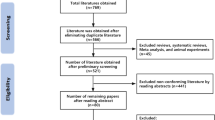
This review was prospectively registered on the PROSPERO register of systematic reviews (CRD42021287850) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. An electronic search of CENTRAL, Embase, Medline, PyscInfo, and Web of Science was performed in November 2021 using the search strategy shown in Fig. 1. Our search strategy utilized Boolean operators of AND/OR to concatenate search terms. Search string syntax was adapted to different databases as necessary, and dates were not restricted. We also manually searched reference lists of included publications to identify potentially eligible studies.
Inclusion/exclusion criteria
Original research articles were included if: (1) the article was available in English; (2) the study was a randomized controlled trial; (3) participants were ≥ 18 years attending a population-based cancer screening program for colorectal cancer OR have previously undergone investigations for colorectal cancer; (4) an individual-level intervention was delivered that addressed one or more of the following colorectal-cancer preventive lifestyle behaviors: physical activity, diet (e.g., red meat or fruit/vegetable consumption, fat consumption), smoking, and alcohol consumption; (5) the intervention was implemented at the time of colorectal cancer screening, or explicitly focused on the reduction of colorectal cancer risk through behavior change; and (6) the study has a control group that either does not receive an intervention or receives treatment distinctly different from the intervention group.
Articles were excluded if they were: (1) not available in English; (2) qualitative studies, literature reviews, or protocol papers; (3) interventions that sought to affect either attendance or participation in colorectal cancer screening programs; (4) interventions that did not seek to change lifestyle-related behaviors such as physical activity, diet, smoking, and alcohol consumption; (5) interventions measuring changes in lifestyle behaviors through qualitative analysis; and (6) interventions that did not have a control group.
Outcomes
In this review, outcomes of interest are changes in health-related lifestyle behaviors associated with risk of colorectal cancer incidence between baseline and last available follow-up among eligible intervention participants. This includes changes in dietary habits, body mass index (BMI), smoking behaviors, alcohol consumption, and physical activity from intervention baseline to the last available follow-up. Each of these outcomes are risk factors associated with the prevention of CRC [12, 13]. We excluded outcomes focused on screening participation, adherence, attendance, engagement, or screening-related behavioral interventions with no concrete behavioral change action.
Study selection
Identified studies from the search databases were downloaded into Endnote and exported to Rayyan. The titles and abstracts of a random selection of 10% of the papers were independently assessed by two or more members of the review team against the listed inclusion and exclusion criteria (VS, GG, DX). After review and discussion, the remaining 90% of titles and abstracts were assessed by two members of the review team, with disagreements being resolved in the presence of a third reviewer (VS, GG, DX). Studies whose title and abstract were not relevant were rejected, and those that cannot be categorized definitively underwent a full text review.
Next, three reviewers (VS, GG, DX) assessed full-text articles against inclusion and exclusion criteria and met regularly to discuss results. Papers that did not meet criteria at this stage will were also excluded. Throughout the selection process, reviewers met regularly to discuss findings and any irregularities observed. Any disagreements during full-text review and data extraction were resolved in the presence of a third reviewer (SG, JUS).
Data extraction
The following data fields were extracted from all studies meeting inclusion criteria: study design, information on participants, information on the intervention (including the components within the TIDieR checklist), behavior change techniques applied during intervention, intervention outcomes, and results [14]. Where studies followed up participants at multiple time points, we extracted data from the longest available follow-up. A data extraction template was built using Microsoft Excel and was piloted by 3 researchers with a small set of full-text articles to resolve any conflicts. Data extraction was completed by a primary reviewer and checked by a secondary reviewer. As such, data extraction for each paper was reviewed by a minimum of two authors.
Behavior change techniques were coded using the Michie et al. taxonomy of 93 BCTs [15]. Two individual pilots for BCT coding were done, where in each pilot one publication was coded by three authors each (VS, GG, DX). This was completed to establish consensus in coding procedures and familiarity with the Michie et al. taxonomy. The remaining publications were coded independently by two authors, with a third author resolving any disagreements (VS, GG, DX).
Risk of bias
Risk of bias was examined using the Cochrane Risk of Bias tool (ROB2), and all studies were examined by two independent reviewers as per Cochrane recommendations [16]. We focused on the following domains pertaining to bias: bias arising from the randomization process; bias due to deviations from intended interventions; bias due to missing outcome data; bias in measurement of the outcome; bias in selection of the reported result.
For each study, the ROB2 tool was used to assess risk of bias using the study’s primary outcome. For each domain, a series of signaling questions (Yes; Probably yes; Probably no; No; No information) were applied to conclude judgements for each domain. The overall risk of bias was expressed as ‘low,’ ‘high,’ or ‘some concerns.’
Data synthesis
Data from the selected interventions were characterized using the TIDieR checklist and coded using the specific BCTs they apply [14]. Regarding measures of treatment effect, measures of primary outcomes (physical activity, diet, alcohol consumption, smoking) were analyzed separately. For interventions with outcome data presented after the intervention concludes, outcomes were reported for the longest follow-up period.
Effect sizes were summarized using forest plots. Effect sizes for dichotomous data were expressed as odds ratios and for continuous outcomes were converted to standardized mean differences (SMD) to account for differences in measurement strategies between interventions. When data for the same outcome were provided as dichotomous data by some studies and as continuous data by others, log odds ratios were converted to SMDs using the methods outlined by Chinn [17].
We combined SMDs across studies for each specific outcome using inverse variance-weighted meta-analysis with each study providing a single effect estimate. Effect sizes were pooled with 95% confidence intervals using a random effects model, and heterogeneity was assessed via a chi-squared test and the I2 statistic. For the I2 statistic, we considered a value of 50% or greater to represent substantial heterogeneity. No meta-regressions were performed due to a low number of available studies for each outcome represented, as per the Cochrane Handbook’s recommendations [18, 19].
Results
We screened 25,087 titles/abstracts for initial eligibility, of which 92 full-text articles met the criteria for additional screening. From this, a total of 10 articles met all inclusion criteria and are included in this review (Fig. 2).
Table 1 provides the details of the included studies, with a focus on the interventions delivered. Of the included studies, six took place in the context of a national CRC screening program [8,9,10, 20,21,22], two took place in the context of screening programs at private hospitals/clinics [23, 24], and two occurred through the context of workplaces [25, 26]. Three of the ten studies included populations that had received/undergone previous investigations for CRC, primarily removal of bowel polyps [22,23,24]. The majority of the studies took place in England, with the second most frequent location being in the United States.
Seven of the studies addressed more than one specific lifestyle behavior, while three focused on only either fruit/vegetable consumption or physical activity. The median length of interventions was nine months with a range of 1.5 to 36 months. The median intervention sample size was 347 with the range being 16 to 3477.
“Int” refers to the intervention group and “Cntr” refers to the control group within a randomized controlled trial.
Risk of bias
Of the ten studies included in this review, three were determined to have low risk of bias, five were determined to have some concerns, and two were determined to have high risk of bias. The greatest source of bias in the included studies was bias from randomization, as nearly half of the included studies did not apply specific allocation sequences or computer-generated programs for randomizing participants. Judgements on each of the five domains of potential bias as per the ROB2 tool are shown below in Fig. 3.
Outcomes
The results of the meta-analysis for each outcome are shown in Fig. 4.
Fruit/Vegetable consumption
Fruit/vegetable consumption was measured in seven of the ten included studies. To measure fruit/vegetable consumption, studies used the Dietary Instrument for Nutrition Education (DINE) [9, 20], the National Cancer Institute Food Frequency Questionnaire (NCI FFQ) [26], the Norwegian LSQ instrument [8], and a two-item questionnaire by Carpuccio et al. [21]. Pooled data from the seven included studies showed a greater increase in fruit/vegetable consumption in the intervention group with respect to the control (SMD 0.13, 95% CI 0.08 to 0.18; p < 0.001).
Physical activity
Similar to fruit/vegetable consumption, seven of the ten included studies measured physical activity. To measure physical activity, studies used accelerometry and pedometer data [21, 22, 24], and self-report measures including the Scottish Physical Activity Questionnaire [20], the Norwegian LSQ [8], the CHAMPS questionnaire [25], and the International Physical Activity Questionnaire (IPAQ) [22]. Our meta-analysis showed no difference in physical activity between the intervention groups compared with controls.
Fiber consumption
Four studies reported outcomes on fiber consumption. To measure fiber consumption, studies used biochemical testing of stool samples [23] and both the DINE [20, 21] and NCI FFQ instruments [26]. The DINE fiber scores differ from the DINE score for fruit/vegetable consumption and ranges from 3 to 88 (arbitrary units) with a score of less than 30 (low) corresponding to a fiber intake of ≤ 20 g/day, and a score of more than 40 (high) corresponding to ≥ 30 g/day. Pooled data from the four included studies showed a greater increase in fiber consumption in the intervention group with respect to the control (SMD 0.14, 95% CI 0.08 to 0.20; p < 0.001).
Alcohol consumption
Four studies reported outcomes on alcohol consumption. Included studies measured alcohol consumption via self-report measures such as the NCI FFQ [25], Norwegian LSQ [8], and the Alcohol Use Disorders Inventory Test [21]. Pooled data from the four included studies showed a greater decrease in alcohol consumption in the intervention group with respect to the control group (SMD 0.19, 95% CI 0.11 to 0.27; p < 0.001).
Smoking behaviors
Two studies reported outcomes on smoking behaviors. Similar to measurements on alcohol consumption behaviors, smoking behaviors were measured via self-report using the NCI FFQ [25] and Norwegian LSQ [8]. Data from the two studies showed a showed a greater proportion of individuals not smoking in the intervention group with respect to the control group (SMD 0.24, 95% CI 0.15 to 0.34; p < 0.001).
Fat consumption
Three studies reported outcomes on fat consumption. Fat consumption was measured through self-report via the NCI FFQ and DINE instruments [21, 26], or via nutrient analysis of stool samples [23]. Pooled data from the three included studies showed a greater decrease in fat consumption in the intervention group with respect to the control, however, the strength of the effect size is smaller than that of other outcomes (SMD 0.09, 95% CI 0.03 to 0.15; p = 0.004).
Body mass index
Two studies reported outcomes body weight. Body mass index was measured through either self-reported or research team measured weight in kilograms and height in centimeters. Pooled data from the two included studies showed no difference in body between the intervention groups compared with controls (SMD 0.03, 95% CI -0.19 to 0.24; p = 0.81).
Multivitamin consumption + red meat consumption
Multivitamin and red meat consumption were each measured by one study. Multivitamin consumption was measured via a single-item self-reported response modified from the Nurse’s Health Study [25], and the SMD of 0.53 indicates a moderate effect size given Cohen’s criteria. Red meat consumption was measured by the semi-quantitative FFQ [25] and had a low effect size with an SMD of 0.24 between those in the intervention group and those in the control group.
Heterogeneity across outcomes
Across all outcomes of interest, we saw moderate to high between-study heterogeneity, evaluated through the I2 value. Between-study heterogeneity varied from 46% among studies measuring alcohol consumption to those measuring fiber consumption and fat consumption, both of which were 93%. Specific values for each outcome are shown below in Fig. 4.
Application of behavior change techniques
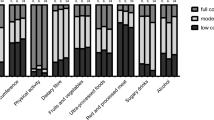
Each included study leveraged behavior change techniques from the Michie et al. taxonomy [15]. As shown in Fig. 5, the median number of BCTs applied by the included studies was 7.5, with a range of two to 13. The most common BCTs applied across the interventions were “feedback on behaviour,” “goal setting,” “information about health consequences,” and “review behavioural goals.”
The application of behavior change techniques was far more common among interventions with in-person coaching or demonstration components. For example, each intervention with more than 10 BCTs applied had one-on-one or group coaching services offered during the intervention. Additionally, interventions that reinforced mechanisms of habit tracking, such as self-weighing and pedometer use [21, 22, 24], applied more BCTs than interventions that solely focused on didactic learning. Interventions which used leaflets/mailings as the primary form of delivery applied the fewest BCTs.
While a meta-regression was not completed in this study, we observed no consistent pattern between the number of behavior change techniques applied and the effect sizes of the studies across our outcomes of interest.
Discussion
Screening programs have been shown to be an effective means of reducing CRC disease burden, and a growing body of literature has described colorectal cancer screening as a “teachable moment” for behavior change [7, 27, 28]. Our findings suggest that interventions seeking to change behaviors related to diet, physical activity, alcohol consumption, and smoking achieved positive, albeit modest, improvements in these behaviors compared with usual care. We additionally no found evidence that a greater number of BCTs was associated with greater behavior change.
While improvements in lifestyle behavior uptake at the individual level were modest, they represent potentially important differences at a population level. For example, the pooled effect size of 0.13 for fruit/vegetable consumption corresponds to an additional 0.3 – 0.6 servings of fruits/vegetables per day [20, 21]. At a population level, if sustained, this increase in fruit/vegetable consumption is estimated to be associated with between a 3 and 6% reduction in CRC risk [29]. Similarly, the pooled effect size for fiber consumption of 0.13 corresponds to a 1.6 g increase in daily fiber intake. Given that each 10 g of fiber consumed daily results in a 7% reduction in CRC risk, this change in behavior, if sustained, corresponds to a 1.12% decrease in population-wide CRC risk [30]. We found similar results for consumption of alcohol and fat. An effect size of 0.09 for fat consumption corresponds to a reduction in daily fat intake of approximately 2.1 g [21, 23]. Given the instruments used, it was not possible to compare pooled effect sizes for alcohol consumption.
Effect sizes for smoking, multivitamin consumption, and red meat consumption similarly all favored the intervention groups. However, the number of studies measuring each outcome was fewer than that for other pooled outcomes. Nevertheless, these results are also potentially promising at the population level. Most notably, previous literature from the World Health Organization suggests that eating 50 g of processed meat daily increases the risk of colorectal cancer by 18% [31], and Botteri et. al suggests that regular smoking increases CRC risk by 15–20% [32].
Although some interventions demonstrated moderate increases in physical activity compared with the respective controls, particularly the three studies including self-monitoring [21, 22, 24], overall the pooled effect sizes show no evidence of change in physical activity. One potential reason for this is the lack of self-monitoring processes included within the other interventions, which were shown to be critical to physical activity behavior change across other interventions. The lack of self-monitoring across interventions is demonstrated in Fig. 4 where it is applied in only three studies [33]. Similar results were concluded for body mass index, which showed no evidence of change even though it has been identified as a risk factor for CRC. Future interventions should further consider how to address body mass index and body weight given its association with CRC risk, and additionally measure body mass index as an outcome especially among studies addressing diet and physical activity.
Previous literature has linked the application of behavior change techniques to positive lifestyle behavior change among individuals eligible for CRC screening [33,34,35,36,37]. Within this study, a meta-regression was not possible due to the limited number of studies, so we were unable to directly quantify the association between applying BCTs and the effectiveness of interventions. However, we found that interventions with a greater number of in-person contacts and tailored information provision applied a greater number of BCTs. The BCTs most frequently utilized among included studies, such as feedback on behavior, goal setting, problem solving, and information on health consequences, have been commonly applied in lifestyle behavior change interventions outside of CRC screening [33, 38]. Future interventions exploring behavior change within this context should explore applying these specific BCTs. However, as we identified no direct relationship between the application of BCTs and the observed effect sizes, additional work is needed to explore the value of BCTs specifically within the context of CRC screening [33,34,35,36,37,38].
Given the BCTs identified within this review, one avenue to further promote lifestyle behavior change within the context of CRC screening is to increase both the level of participant engagement and the effectiveness of existing engagement within interventions [39, 40]. One avenue may be the application of digital components or tools, especially given the existing literature supporting the effectiveness of such interventions [41, 42]. For example, a number of publications have cited the benefit of applying digital tools to improve the frequency, duration, and tailoring of engagement along with the depth of engagement within behavior change interventions [43,44,45]. However, within the context of CRC screening, additional considerations must be made to the effect of participant age within digital interventions. While digital interventions applying BCT’s focused on older populations have shown initial success, further evidence is needed to draw conclusions surrounding efficacy [47,48,49]. Specifically, opportunities to engage patients over longer periods of time as means to promote sustained behavior change are needed, especially those that can achieve outcomes in a cost-effective manner.
The findings of this systematic review have significant relevance among the broader body of literature promoting lifestyle behavior change as a means of reducing cancer risk. Across several lifestyle behaviors, this review quantified the impact of specific interventions on CRC risk, which while small in magnitude, are significant on a population level. Orange et al. highlighted similar conclusions across both colorectal and breast cancer, and numerous other studies have shown the association between lifestyle behaviors such as diet, physical activity, smoking, and red meat consumption and cancer risk [50,51,52,53,54,55]. However, additional work is needed applying similar interventions to other cancers, and secondarily contextualizing the value of screening as an opportunity for health promotion.
This review has several strengths that build on limitations of previous reviews investigating the effectiveness of behavior change interventions implemented during CRC screening. First, all articles meeting initial database search criteria were double screened. Second, this review includes interventions whose participants have undergone previous investigations for CRC, allowing us to evaluate interventions that may be applicable to the context of screening itself. This is especially relevant within the context of national CRC screening programs, which have multiple stages and thus require longer-term engagement across heterogeneous patient population. Furthermore, characterizing the BCTs applied across interventions provides valuable insight into how future interventions could better integrate behavioral science principles to target specific behavior change outcomes. Additionally, the majority of interventions within this review (7/10) had clinical professionals involved with intervention delivery, indicating a focus on implementation within clinical practice.
Additionally, this review has several limitations. Within each outcome in the meta-analysis, we observed moderate to high levels of between-study heterogeneity, and 70% of the included studies had “some concerns” or “high risk” of bias as per the Cochrane ROB2 tool primarily due to the lack of robust randomization methods applied. The between-study heterogeneity may be due to the fact that many of the primary measurement strategies across these outcomes were self-report and recall measures of limited rigor with different lengths of follow-up measurements. For example, to measure physical activity, several studies used less rigorous self-report measures as compared to more objective measures such as pedometer data [21, 22, 24]. To reduce bias and error, future interventions measuring change in lifestyle behaviors should seek to implement more objective measurement strategies. Many of the studies included in this review were conducted either in the United Kingdom or United States, and as such, the results may also not be directly applicable to the contexts of other healthcare systems around the world. Three studies measured outcomes for less than 6 months, limiting our understanding of the long-term effectiveness of such interventions. Lastly, we only included studies in English in this review, which may limit the identification of several studies in gray literature and additional peer-reviewed literature in other languages.
Conclusion
In this systematic review we have identified positive improvements in fruit/vegetable consumption, fiber consumption, fat consumption, red meat and multivitamin consumption, alcohol consumption, and smoking behaviors following interventions either delivered within CRC screening program or targeted specifically at reducing CRC risk among those eligible for CRC screening. While the magnitude of the pooled effect sizes varied, they correspond to potentially important changes in lifestyle behaviors when considered on a population scale. Interventions with more in-person engagement between participants and trial staff applied a greater number of BCTs, however we saw no pattern between the number of BCTs applied and the observed effect size. Together with evidence from previous work, our findings indicate that interventions delivered at the time of screening or shortly afterward represents a promising opportunity to reduce CRC risk through behavior change. Future interventions should identify avenues to maximize long-term engagement to promote sustained lifestyle behavior change.
References
Soriano LC, Soriano-Gabarró M, García Rodríguez LA (2018) Trends in the contemporary incidence of colorectal cancer and patient characteristics in the United Kingdom: a population-based cohort study using The Health Improvement Network. BMC Cancer 18:402. https://doi.org/10.1186/s12885-018-4265-1
Aune D, Lau R, Chan DS et al (2011) Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology 141:106–118
Boyle T, Keegel T, Bull F et al (2012) Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst 104:1548–1561
Ma Y, Yang Y, Wang F et al (2013) Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS ONE 8:e53916
Bevan R, Rutter MD (2018) Colorectal cancer screening-who, how, and when? Clin Endosc 51(1):37–49. https://doi.org/10.5946/ce.2017.141
Koo S, Neilson LJ, Von Wagner C, Rees CJ (2017) The NHS Bowel Cancer Screening Program: current perspectives on strategies for improvement. Risk management and healthcare policy 10:177–187. https://doi.org/10.2147/RMHP.S109116
Anderson AS, Mackison D, Boath C, Steele R (2013) Promoting changes in diet and physical activity in breast and colorectal cancer screening settings: an unexplored opportunity for endorsing healthy behaviors. Cancer Prev Res 6:165–172
Knudsen MD, Hjartåker A, Robb KA, de Lange T, Hoff G, Berstad P (2018) Improving cancer preventive behaviors: a randomized trial of tailored lifestyle feedback in colorectal cancer screening. Cancer Epidemiol Biomark Prev 27(12):1442–1449. https://doi.org/10.1158/1055-9965.EPI-18-0268
Baker AH, Wardle J (2002) Increasing fruit and vegetable intake among adults attending colorectal cancer screening: the efficacy of a brief tailored intervention. Cancer Epidemiol Biomark Prev 11(2):203–206
Robb KA, Power E, Kralj-Hans I, Atkin WS, Wardle J (2010) The impact of individually-tailored lifestyle advice in the colorectal cancer screening context: a randomised pilot study in North-West London. Prev Med 51(6):505–508. https://doi.org/10.1016/j.ypmed.2010.10.002
Orange ST, Hicks KM, Saxton JM (2021) Effectiveness of diet and physical activity interventions amongst adults attending colorectal and breast cancer screening: a systematic review and meta-analysis. Cancer Causes Control 32:13–26. https://doi.org/10.1007/s10552-020-01362-5
Lewandowska A, Rudzki G, Lewandowski T, Stryjkowska-Góra A, Rudzki S (2022) Title: risk factors for the diagnosis of colorectal cancer. Cancer Control 29:10732748211056692. https://doi.org/10.1177/10732748211056692
Centers for Disease Control and Prevention. (2022, February 17). What are the risk factors for colorectal cancer? Centers for Disease Control and Prevention. Retrieved June 1, 2022, from https://www.cdc.gov/cancer/colorectal/basic_info/risk_factors.htm
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan AW, Michie S (2014) Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 348:g1687. https://doi.org/10.1136/bmj.g1687
Michie S, Richardson M, Johnston M et al (2013) The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann Behav Med 46:81–95
Rob 2: A revised Cochrane Risk-of-bias tool for randomized trials. RoB 2: A revised Cochrane risk-of-bias tool for randomized trials | Cochrane Bias. (n.d.). Retrieved June 2, 2022, from https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials
Chinn S (2000) A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med 19(22):3127–3131. https://doi.org/10.1002/1097-0258(20001130)19:22%3c3127::aid-sim784%3e3.0.co;2-m
Meta Regression. 9.6.4 meta-regression. (n.d.). Retrieved June 2, 2022, from https://handbook-5-1.cochrane.org/chapter_9/9_6_4_meta_regression.htm
Geissbühler M, Hincapié CA, Aghlmandi S, Zwahlen M, Jüni P, da Costa BR (2021) Most published meta-regression analyses based on aggregate data suffer from methodological pitfalls: a meta-epidemiological study. BMC Med Res Methodol 21(1):123. https://doi.org/10.1186/s12874-021-01310-0
Caswell S, Anderson AS, Steele RJ (2009) Bowel health to better health: a minimal contact lifestyle intervention for people at increased risk of colorectal cancer. Br J Nutr 102(11):1541–1546. https://doi.org/10.1017/S0007114509990808
Anderson AS, Craigie AM, Caswell S, Treweek S, Stead M, Macleod M, Daly F, Belch J, Rodger J, Kirk A, Ludbrook A, Rauchhaus P, Norwood P, Thompson J, Wardle J, Steele RJ (2014) The impact of a bodyweight and physical activity intervention (BeWEL) initiated through a national colorectal cancer screening programme: randomised controlled trial. BMJ (Clinical Research Ed) 348:g1823. https://doi.org/10.1136/bmj.g1823
Lewis LS, Shaw B, Banerjee S, Dieguez P, Hernon J, Belshaw N, Saxton JM (2020) The role of self-determination in changing physical activity behavior in people diagnosed with bowel polyps: a pilot randomized controlled trial. J Aging Phys Act 28(1):42–52. https://doi.org/10.1123/japa.2018-0279
McKeown-Eyssen GE, Bright-See E, Bruce WR, Jazmaji V, Cohen LB, Pappas SC, Saibil FG (1994) A randomized trial of a low fat high fibre diet in the recurrence of colorectal polyps. Toronto Polyp Prevention Group. Journal of clinical epidemiology 47(5):525–536. https://doi.org/10.1016/0895-4356(94)90299-2
Wolin KY, Fagin C, James AS, Early DS (2012) Promoting physical activity in patients with colon adenomas: a randomized pilot intervention trial. PLoS ONE 7(7):e39719. https://doi.org/10.1371/journal.pone.0039719
Emmons KM, McBride CM, Puleo E, Pollak KI, Clipp E, Kuntz K, Marcus BH, Napolitano M, Onken J, Farraye F, Fletcher R (2005) Project PREVENT: a randomized trial to reduce multiple behavioral risk factors for colon cancer. Cancer Epidemiol Biomark Prev 14(6):1453–1459. https://doi.org/10.1158/1055-9965.EPI-04-0620
Tilley BC, Glanz K, Kristal AR, Hirst K, Li S, Vernon SW, Myers R (1999) Nutrition intervention for high-risk auto workers: results of the Next Step Trial. Prev Med 28(3):284–292. https://doi.org/10.1006/pmed.1998.0439
Stevens C, Smith SG, Vrinten C, Waller J, Beeken RJ (2019) Lifestyle changes associated with participation in colorectal cancer screening: Prospective data from the english longitudinal study of ageing. J Med Screen 26(2):84–91. https://doi.org/10.1177/0969141318803973
Lee AS, Ozakinci G, Leung S et al (2016) Lifestyle change in the cancer setting using ‘the teachable moment’: protocol for a proof-of-concept pilot in a urology service. Pilot Feasibility Stud 2:65. https://doi.org/10.1186/s40814-016-0102-y
Consumption of Fruits and Vegetables and Risk of Colorectal Adenoma: A PRISMA-Compliant Meta-Analysis of Observational Studies. Medicine, 94(42), e1599. https://doi.org/10.1097/MD.0000000000001599
Ma, Y., Hu, M., Zhou, L., Ling, S., Li, Y., Kong, B., & Huang, P. (2018). Dietary fiber intake and risks of proximal and distal colon cancers: A meta-analysis. Medicine, 97(36), e11678. https://doi.org/10.1097/MD.0000000000011678
World Health Organization. (n.d.). Cancer: Carcinogenicity of the consumption of red meat and processed meat. World Health Organization. Retrieved June 3, 2022, from https://www.who.int/news-room/questions-and-answers/item/cancer-carcinogenicity-of-the-consumption-of-red-meat-and-processed-meat#:~:text=An%20analysis%20of%20data%20from,cancer%20is%20not%20as%20strong.
Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P (2008) Smoking and colorectal cancer: a meta-analysis. JAMA 300(23):2765–2778. https://doi.org/10.1001/jama.2008.839
Samdal GB, Eide GE, Barth T et al (2017) Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. Int J Behav Nutr Phys Act 14:42. https://doi.org/10.1186/s12966-017-0494-y
Gardner B, Smith L, Lorencatto F, Hamer M, Biddle SJ (2016) How to reduce sitting time? A review of behaviour change strategies used in sedentary behaviour reduction interventions among adults. Health Psychol Rev 10(1):89–112. https://doi.org/10.1080/17437199.2015.1082146
Cradock KA, ÓLaighin, G., Finucane, F. M., Gainforth, H. L., Quinlan, L. R., & Ginis, K. A. (2017) Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: a systematic review and meta-analysis. Int J Behav Nutr Phys Activ 14(1):18. https://doi.org/10.1186/s12966-016-0436-0
French DP, Olander EK, Chisholm A, Mc Sharry J (2014) Which behaviour change techniques are most effective at increasing older adults’ self-efficacy and physical activity behaviour? A systematic review. Ann Behav Med 48(2):225–234. https://doi.org/10.1007/s12160-014-9593-z
Lara J, Evans EH, O’Brien N et al (2014) Association of behaviour change techniques with effectiveness of dietary interventions among adults of retirement age: a systematic review and meta-analysis of randomised controlled trials. BMC Med 12:177. https://doi.org/10.1186/s12916-014-0177-3
Howlett N, Trivedi D (2019) Nicholas A Troop, Angel Marie Chater, Are physical activity interventions for healthy inactive adults effective in promoting behavior change and maintenance, and which behavior change techniques are effective? A systematic review and meta-analysis. Transl Behav Med 9(1):147–157. https://doi.org/10.1093/tbm/iby010
Krist AH, Tong ST, Aycock RA, Longo DR (2017) Engaging patients in decision-making and behavior change to promote prevention. Stud Health Technol Inform 240:284–302
Young C, Campolonghi S, Ponsonby S, Dawson SL, O’Neil A, Kay-Lambkin F, McNaughton SA, Berk M, Jacka FN (2019) Supporting engagement, adherence, and behavior change in online dietary interventions. J Nutr Educ Behav 51(6):719–739. https://doi.org/10.1016/j.jneb.2019.03.006
Yardley L, Spring B, Morrison L, Crane D, Curtis K, Merchant G, Naughton F, Blandford A (2016) Understanding and promoting effective engagement with digital behavior change interventions. Am J Prev Med 51:833–842. https://doi.org/10.1016/j.amepre.2016.06.015
Perski O, Blandford A, West R, Michie S (2017) Conceptualising engagement with digital behaviour change interventions: a systematic review using principles from critical interpretive synthesis. Transl Behav Med 7(2):254–267. https://doi.org/10.1007/s13142-016-0453-1
Michie S, Yardley L, West R, Patrick K, Greaves F (2017) Developing and evaluating digital interventions to promote behavior change in health and health care: recommendations resulting from an international workshop. J Med Internet Res 19(6):e232. https://doi.org/10.2196/jmir.7126
Dennison L, Morrison L, Conway G, Yardley L (2013) Opportunities and challenges for smartphone applications in supporting health behavior change: qualitative study. J Med Internet Res 15(4):e86. https://doi.org/10.2196/jmir.2583
Chen Y, Perez-Cueto F, Giboreau A, Mavridis I, Hartwell H (2020) The promotion of eating behaviour change through digital interventions. Int J Environ Res Public Health 17(20):7488. https://doi.org/10.3390/ijerph17207488
Andrade C (2020) Mean difference, standardized mean difference (smd), and their use in meta-analysis: as simple as it gets. J Clin Psychiatr 81(5):20f13681. https://doi.org/10.4088/JCP.20f13681
Boucher E, Honomichl R, Ward H, Powell T, Stoeckl SE, Parks A (2022) The Effects of a Digital Well-being Intervention on Older Adults: Retrospective Analysis of Real-world User Data. JMIR Aging 5(3):e39851. https://doi.org/10.2196/39851
Stockwell S, Schofield P, Fisher A, Firth J, Jackson SE, Stubbs B, Smith L (2019) Digital behavior change interventions to promote physical activity and/or reduce sedentary behavior in older adults: a systematic review and meta-analysis. Exp Gerontol 120:68–87. https://doi.org/10.1016/j.exger.2019.02.020
Riadi I, Kervin L, Dhillon S, Teo K, Churchill R, Card KG, Sixsmith A, Moreno S, Fortuna KL, Torous J, Cosco TD (2022) Digital interventions for depression and anxiety in older adults: a systematic review of randomised controlled trials. Lancet Healthy longevity 3(8):e558–e571. https://doi.org/10.1016/S2666-7568(22)00121-0
McTiernan A, Friedenreich CM, Katzmarzyk PT, Powell KE, Macko R, Buchner D, Pescatello LS, Bloodgood B, Tennant B, Vaux-Bjerke A, George SM, Troiano RP, Piercy KL, Physical Activity Guidelines Advisory Committee (2019) Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc 51(6):1252–1261. https://doi.org/10.1249/MSS.0000000000001937
Winzer BM, Whiteman DC, Reeves MM, Paratz JD (2011) Physical activity and cancer prevention: a systematic review of clinical trials. Cancer causes & control : CCC 22(6):811–826. https://doi.org/10.1007/s10552-011-9761-4
Jia T, Liu Y, Fan Y, Wang L, Jiang E (2022) Association of healthy diet and physical activity with breast cancer: lifestyle interventions and oncology education. Front Publ Health 10:797794. https://doi.org/10.3389/fpubh.2022.797794
Yoo JE, Han K, Shin DW, Jung W, Kim D, Lee CM, Kwon H, Jung KW, Song YM (2022) Effect of smoking reduction, cessation, and resumption on cancer risk: a nationwide cohort study. Cancer 128(11):2126–2137. https://doi.org/10.1002/cncr.34172
Chang JT, Anic GM, Rostron BL, Tanwar M, Chang CM (2021) Cigarette smoking reduction and health risks: a systematic review and meta-analysis. Nicot Tobacco Res 23(4):635–642. https://doi.org/10.1093/ntr/ntaa156
Ubago-Guisado E, Rodríguez-Barranco M, Ching-López A, Petrova D, Molina-Montes E, Amiano P, Barricarte-Gurrea A, Chirlaque MD, Agudo A, Sánchez MJ (2021) Evidence update on the relationship between diet and the most common cancers from the european prospective investigation into cancer and nutrition (EPIC) study: a systematic review. Nutrients 13(10):3582. https://doi.org/10.3390/nu13103582
Acknowledgments
The authors would like to thank Stephen Sharp from the University of Cambridge MRC Epidemiology Unit for his contribution to the statistical methods applied in this work, and Isla Kuhn from the University of Cambridge Medical Library for her contribution to our database search strategy.
Funding
This work was supported, in whole or part, by the Bill & Melinda Gates Foundation [OPP1144]. Under the grand conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
Author information
Authors and Affiliations
Contributions
Veeraj Shah, Lily Taylor, Juliet Usher-Smith, and Simon Griffin contributed to the review’s goals, research questions, and design. Veeraj Shah, Greta Geller, and Diane Xu contributed to the screening of articles from initial title/abstract screening to full text review, along with data extraction. The first draft of the manuscript was written by Veeraj Shah and all authors contributed to interpretation of the findings and critically revised previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This is a systematic review, as such no ethical approval was required to complete this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shah, V., Geller, G., Xu, D. et al. Evaluating the potential impact of lifestyle-based behavior change interventions delivered at the time of colorectal cancer screening. Cancer Causes Control 35, 561–574 (2024). https://doi.org/10.1007/s10552-023-01773-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-023-01773-0