Abstract
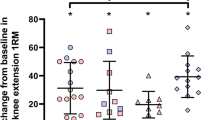
Resistance training (RT) can increase the heat shock response (HSR) in the elderly. As middle-aged subjects already suffer physiological declines related to aging, it is hypothesized that RT may increase the HSR in these people. To assess the effects of resistance training on heat shock response, intra and extracellular HSP70, oxidative stress, inflammation, body composition, and metabolism in middle-aged subjects. Sixteen volunteers (40 – 59 years) were allocated to two groups: the trained group (n = 7), which performed 12 weeks of RT; and the physically inactive—control group (n = 9), which did not perform any type of exercise. The RT program consisted of 9 whole-body exercises (using standard gym equipment) and functional exercises, carried out 3 times/week. Before and after the intervention, body composition, muscle mass, strength, functional capacity, and blood sample measurements (lipid profile, glucose, insulin, oxidative damage, TNF-α, the HSR, HSP70 expression in leukocytes, and HSP72 in plasma) were performed. The HSR analysis demonstrated that this response is maintained at normal levels in middle-aged people and that RT did not cause any improvement. Also, RT increases muscle mass, strength, and functional capacity. Despite no additional changes of RT on the antioxidant defenses (catalase, glutathione peroxidase, and reductase) or inflammation, lipid peroxidation was diminished by RT (group x time interaction, p = 0.009), indicating that other antioxidant defenses may be improved after RT. HSR is preserved in middle-aged subjects without metabolic complications. In addition, RT reduces lipid peroxidation and can retard muscle mass and strength loss related to the aging process.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Alikhani S, Sheikholeslami-Vatani D (2019) Oxidative stress and anti-oxidant responses to regular resistance training in young and older adult women. Geriatr Gerontol Int 19:419–422. https://doi.org/10.1111/ggi.13636
Bautmans I, Njemini R, Vasseur S, Chabert H, Moens L et al (2005) Biochemical changes in response to intensive resistance exercise training in the elderly. Gerontology 51:253–265. https://doi.org/10.1159/000085122
Bay ML, Pedersen BK (2020) Muscle-organ crosstalk: focus on immunometabolism. Front Physiol 11:567881. https://doi.org/10.3389/fphys.2020.567881
Beltran Valls MR, Dimauro I, Brunelli A, Tranchita E, Ciminelli E et al (2014) Explosive type of moderate-resistance training induces functional, cardiovascular, and molecular adaptations in the elderly. Age (Dordr) 36:759–772. https://doi.org/10.1007/s11357-013-9584-1
Borges Russo MK, Kowalewski LS, da Natividade GR, de Lemos Muller CH, Schroeder HT et al (2022) Elevated extracellular HSP72 and blunted heat shock response in severe COVID-19 patients. Biomolecules 12. https://doi.org/10.3390/biom12101374
Calderwood SK, Murshid A, Prince T (2009) The shock of aging: molecular chaperones and the heat shock response in longevity and aging–a mini-review. Gerontology 55:550–558. https://doi.org/10.1159/000225957
Costamagna D, Costelli P, Sampaolesi M, Penna F (2015) Role of inflammation in muscle homeostasis and myogenesis. Mediators Inflamm 2015:805172. https://doi.org/10.1155/2015/805172
de Lemos Muller CH, Rech A, Botton CE, Schroeder HT, Bock PM et al (2018) Heat-induced extracellular HSP72 release is blunted in elderly diabetic people compared with healthy middle-aged and older adults, but it is partially restored by resistance training. Exp Gerontol 111:180–187. https://doi.org/10.1016/j.exger.2018.07.014
de Lemos Muller CH, de Matos JR, Grigolo GB, Schroeder HT, Rodrigues-Krause J, Krause M (2019) Exercise training for the elderly: inflammaging and the central role for HSP70. J Sci Sport Exerc 1:97–115. https://doi.org/10.1007/s42978-019-0015-6
de Souza DC, Matos VAF, Dos Santos VOA, Medeiros IF, Marinho CSR et al (2018) Effects of high-intensity interval and moderate-intensity continuous exercise on inflammatory, leptin, IgA, and lipid peroxidation responses in obese males. Front Physiol 9:567. https://doi.org/10.3389/fphys.2018.00567
De Maio A, Vazquez D (2013) Extracellular heat shock proteins: a new location, a new function. Shock 40:239–246. https://doi.org/10.1097/SHK.0b013e3182a185ab
Durlak JA (2009) How to select, calculate, and interpret effect sizes. J Pediatr Psychol 34:917–928. https://doi.org/10.1093/jpepsy/jsp004
Farinha JB, Ramis TR, Vieira AF, Macedo RCO, Rodrigues-Krause J et al (2018) Glycemic, inflammatory and oxidative stress responses to different high-intensity training protocols in type 1 diabetes: a randomized clinical trial. J Diabetes Complicat 32:1124–1132. https://doi.org/10.1016/j.jdiacomp.2018.09.008
Fehrenbach E, Passek F, Niess AM, Pohla H, Weinstock C et al (2000) HSP expression in human leukocytes is modulated by endurance exercise. Med Sci Sports Exerc 32:592–600. https://doi.org/10.1097/00005768-200003000-00007
Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M et al (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254. https://doi.org/10.1111/j.1749-6632.2000.tb06651.x
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Galvao DA, Taaffe DR (2005) Resistance exercise dosage in older adults: single- versus multiset effects on physical performance and body composition. J Am Geriatr Soc 53:2090–2097. https://doi.org/10.1111/j.1532-5415.2005.00494.x
Garcia-Lopez D, Hakkinen K, Cuevas MJ, Lima E, Kauhanen A et al (2007) Effects of strength and endurance training on antioxidant enzyme gene expression and activity in middle-aged men. Scand J Med Sci Sports 17:595–604. https://doi.org/10.1111/j.1600-0838.2006.00620.x
Giraldo E, Hinchado MD, Ortega E (2013) Combined activity of post-exercise concentrations of NA and eHsp72 on human neutrophil function: role of cAMP. J Cell Physiol 228:1902–1906. https://doi.org/10.1002/jcp.24354
Grivas GV, Karatrantou K, Chasialis A, Batatolis C, Ioakimidis P, Gerodimos V (2022) Serial vs. integrated outdoor combined training programs for health promotion in middle-aged males. Sports (Basel) 10. https://doi.org/10.3390/sports10080122
Hakkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H et al (1998) Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol 1985(84):1341–1349. https://doi.org/10.1152/jappl.1998.84.4.1341
Hallal PC, Matsudo S, Farias JC Jr (2012) Measurement of physical activity by self-report in low- and middle-income countries: more of the same is not enough. J Phys Act Health 9(Suppl 1):S88-90. https://doi.org/10.1123/jpah.9.s1.s88
Himmelfarb J, McMonagle E, McMenamin E (2000) Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int 58:2571–2578. https://doi.org/10.1046/j.1523-1755.2000.00443.x
Janssen I, Heymsfield SB, Wang ZM, Ross R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol 1985(89):81–88. https://doi.org/10.1152/jappl.2000.89.1.81
Kavanagh K, Wylie AT, Chavanne TJ, Jorgensen MJ, Voruganti VS et al (2012) Aging does not reduce heat shock protein 70 in the absence of chronic insulin resistance. J Gerontol A Biol Sci Med Sci 67:1014–1021. https://doi.org/10.1093/gerona/gls008
Kennis E, Verschueren S, Van Roie E, Thomis M, Lefevre J, Delecluse C (2014) Longitudinal impact of aging on muscle quality in middle-aged men. Age (Dordr) 36:9689. https://doi.org/10.1007/s11357-014-9689-1
Korhonen MT, Mero AA, Alen M, Sipila S, Hakkinen K et al (2009) Biomechanical and skeletal muscle determinants of maximum running speed with aging. Med Sci Sports Exerc 41:844–856. https://doi.org/10.1249/MSS.0b013e3181998366
Krause M, Keane K, Rodrigues-Krause J, Crognale D, Egan B et al (2013) Elevated levels of extracellular heat-shock protein 72 (eHSP72) are positively correlated with insulin resistance in vivo and cause pancreatic beta-cell dysfunction and death in vitro. Clin Sci (Lond) 126:739–752. https://doi.org/10.1042/CS20130678
Krause M, Heck TG, Bittencourt A, Scomazzon SP, Newsholme P et al (2015) The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediators Inflamm 2015:249205. https://doi.org/10.1155/2015/249205
Krause M, Crognale D, Cogan K, Contarelli S, Egan B et al (2018) The effects of a combined bodyweight-based and elastic bands resistance training, with or without protein supplementation, on muscle mass, signaling and heat shock response in healthy older people. Exp Gerontol 115:104–113. https://doi.org/10.1016/j.exger.2018.12.004
Krause M, Gerchman F, Friedman R (2020) Coronavirus infection (SARS-CoV-2) in obesity and diabetes comorbidities: is heat shock response determinant for the disease complications? Diabetol Metab Syndr 12:63. https://doi.org/10.1186/s13098-020-00572-w
Lenhard WLA (2016) Computation of effect sizes. https://www.psychometrica.de/effect_size.html
Lombardi P (ed) (1989) Beginning weight training: the safe and effective way. Brown & Benchmark Pub
Lynch GS (2004) Tackling Australia’s future health problems: developing strategies to combat sarcopenia–age-related muscle wasting and weakness. Intern Med J 34:294–296. https://doi.org/10.1111/j.1444-0903.2004.00568.x
Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC (2010) Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging 14:362–366. https://doi.org/10.1007/s12603-010-0081-2
Martinez de Toda I, De la Fuente M (2015) The role of Hsp70 in oxi-inflamm-aging and its use as a potential biomarker of lifespan. Biogerontology 16:709–721. https://doi.org/10.1007/s10522-015-9607-7
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419. https://doi.org/10.1007/BF00280883
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Nakhjavani M, Morteza A, Khajeali L, Esteghamati A, Khalilzadeh O et al (2010) Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones 15:959–964. https://doi.org/10.1007/s12192-010-0204-z
Njemini R, Abeele MV, Demanet C, Lambert M, Vandebosch S, Mets T (2002) Age-related decrease in the inducibility of heat-shock protein 70 in human peripheral blood mononuclear cells. J Clin Immunol 22:195–205. https://doi.org/10.1023/a:1016036724386
Njemini R, Forti LN, Mets T, Van Roie E, Coudyzer W et al (2017) Sex difference in the heat shock response to high external load resistance training in older humans. Exp Gerontol 93:46–53. https://doi.org/10.1016/j.exger.2017.04.005
Noble EG, Milne KJ, Melling CW (2008) Heat shock proteins and exercise: a primer. Appl Physiol Nutr Metab 33:1050–1065. https://doi.org/10.1139/H08-069
Ogawa K, Sanada K, Machida S, Okutsu M, Suzuki K (2010) Resistance exercise training-induced muscle hypertrophy was associated with reduction of inflammatory markers in elderly women. Mediators Inflamm 2010:171023. https://doi.org/10.1155/2010/171023
Ogawa K, Kim HK, Shimizu T, Abe S, Shiga Y, Calderwood SK (2012) Plasma heat shock protein 72 as a biomarker of sarcopenia in elderly people. Cell Stress Chaperones 17:349–359. https://doi.org/10.1007/s12192-011-0310-6
Perez LM, Pareja-Galeano H, Sanchis-Gomar F, Emanuele E, Lucia A, Galvez BG (2016) “Adipaging”: ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J Physiol 594:3187–3207. https://doi.org/10.1113/JP271691
Perreault K, Courchesne-Loyer A, Fortier M, Maltais M, Barsalani R et al (2016) Sixteen weeks of resistance training decrease plasma heat shock protein 72 (eHSP72) and increase muscle mass without affecting high sensitivity inflammatory markers’ levels in sarcopenic men. Aging Clin Exp Res 28:207–214. https://doi.org/10.1007/s40520-015-0411-7
Phillips BE, Williams JP, Greenhaff PL, Smith K, Atherton PJ (2017) Physiological adaptations to resistance exercise as a function of age. JCI Insight 2. https://doi.org/10.1172/jci.insight.95581
Radaelli R, Wilhelm EN, Botton CE, Rech A, Bottaro M et al (2014) Effects of single vs. multiple-set short-term strength training in elderly women. Age (Dordr) 36:9720. https://doi.org/10.1007/s11357-014-9720-6
Rikli RE, Jones CJ (1999) Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act 7(2):129–161. https://doi.org/10.1123/japa.7.2.129
Robertson RJ, Goss FL, Rutkowski J, Lenz B, Dixon C et al (2003) Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med Sci Sports Exerc 35:333–341. https://doi.org/10.1249/01.MSS.0000048831.15016.2A
Rodriguez-Miguelez P, Fernandez-Gonzalo R, Almar M, Mejias Y, Rivas A et al (2014) Role of Toll-like receptor 2 and 4 signaling pathways on the inflammatory response to resistance training in elderly subjects. Age (Dordr) 36:9734. https://doi.org/10.1007/s11357-014-9734-0
Sankar J, Mohan G, Pariyarath R, Verghese J (2012) Visceral fat assessment in over nourished children by ultrasonography and its relation to anthropometry. Indian J Pediatr 79:1338–1341. https://doi.org/10.1007/s12098-011-0659-7
Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB et al (2009) Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci 64:1183–1189. https://doi.org/10.1093/gerona/glp097
Semiz S, Ozgoren E, Sabir N (2007) Comparison of ultrasonographic and anthropometric methods to assess body fat in childhood obesity. Int J Obes (Lond) 31:53–58. https://doi.org/10.1038/sj.ijo.0803414
Simar D, Malatesta D, Koechlin C, Cristol JP, Vendrell JP, Caillaud C (2004) Effect of age on Hsp72 expression in leukocytes of healthy active people. Exp Gerontol 39:1467–1474. https://doi.org/10.1016/j.exger.2004.08.002
Simar D, Malatesta D, Badiou S, Dupuy AM, Caillaud C (2007) Physical activity modulates heat shock protein-72 expression and limits oxidative damage accumulation in a healthy elderly population aged 60 90 years. J Gerontol A Biol Sci Med Sci 62:1413–1419. https://doi.org/10.1093/gerona/62.12.1413
Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J et al (2010) Fat tissue, aging, and cellular senescence. Aging Cell 9:667–684. https://doi.org/10.1111/j.1474-9726.2010.00608.x
Terry DF, Wyszynski DF, Nolan VG, Atzmon G, Schoenhofen EA et al (2006) Serum heat shock protein 70 level as a biomarker of exceptional longevity. Mech Ageing Dev 127:862–868. https://doi.org/10.1016/j.mad.2006.08.007
Vincent HK, Bourguignon C, Vincent KR (2006) Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity (Silver Spring) 14:1921–1930. https://doi.org/10.1038/oby.2006.224
Acknowledgements
C.H.L.M. and H.T.S. were supported by scholarships from Brazilian National Council for Scientific and Technological Development (CNPq, Brazil). A.R.-O., P.I.H.B.J and M.K. are Research Productivity Fellows of the Brazilian National Council for Scientific and Technological Development (CNPq, Brazil).
Funding
This work was supported by The State of Rio Grande do Sul Foundation for Research Support (FAPERGS; grant #30791.434.41354.23112017—CHAMADA FAPERGS/Decit/SCTIE/ MS/CNPq/SESRS n. 03/2017 – PPSUS, to M.K.), and The Brazilian National Council for Scientific and Technological Development (CNPq; grants #551097/2007–8, 563870/2010–9, 402626/2012–5, and 402364/2012–0, to P.I.H.B.J., and # 404707/2016–5 to A.R-O).
Author information
Authors and Affiliations
Contributions
M.K., R.S.P and A.R-O conceived and designed research. Funding acquisition: P.I.H.B.J., A.R-O. and M.K. Enrollment of subjects, testing and data analysis: C.H.L.M., H.T.S., J.B.F, and P.L. Writing—original draft preparation: C.H.L.M. and M.K. Writing – review, and editing: P.I.H.B.J., A.R-O, R.S.P. All authors read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Ethics Committee of the Universidade Federal do Rio Grande do Sul (protocol number 1.614.907), and was conducted according to the Declaration of Helsinki, except for registration in a database.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
No potential conflict of interest was reported by the author(s).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
- Heat Shock Response is preserved in healthy middle-aged adults and is not improved by resistance training;.
- Resistance training improves oxidative damage without affecting antioxidant defenses or inflammation;.
- Resistance training improves muscle mass and function in middle-aged people but does not alter visceral fat tissue.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Lemos Muller, C.H., Schroeder, H.T., Farinha, J.B. et al. Effects of resistance training on heat shock response (HSR), HSP70 expression, oxidative stress, inflammation, and metabolism in middle-aged people. J Physiol Biochem 80, 161–173 (2024). https://doi.org/10.1007/s13105-023-00994-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-023-00994-w