Abstract
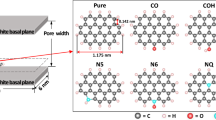
A Grand Canonical Monte Carlo simulation (GCMC) method is used to study the adsorption of methane and carbon dioxide on porous silica glass in the presence and absence of nickel. Nickel atoms are randomly allocated on pore walls, accounting for approximately 1–5% by weight. Experimental data is collected for various nickel concentrations ranging from 0 to 10%. The preparation of porous glass in the presence of Ni is done both with and without calcination in the furnace. The simulation investigates the adsorption of methane and carbon dioxide at temperatures of 273 K and 298 K for different pore widths. The adsorption of methane increases with higher nickel concentration due to the stronger interaction between methane and nickel. However, the opposite behavior is observed in the case of carbon dioxide. Physical adsorption reveals that fluid adsorption on porous glass surfaces decreases as temperature increases. The adsorption of methane begins at the nickel molecule and then progresses further inside the pore. However, in the experimental work, a similar behavior is found for nickel concentrations less than 5% with calcination, after which the adsorption decreases with increasing Ni concentration. This may be attributed to the blocking of Ni molecules at the pore entrance, resulting in difficulty for methane diffusion through the pore. Regarding the effect of Ni allocation on glass surfaces, it is observed that the isotherm obtained for randomly placed nickels is greater than that for nickels placed at the pore edge. This can also be attributed to the pore-blocking effect. The allocation of Ni does not significantly affect the adsorption of carbon dioxide. The finding of this study are supported by similarity to the results reported by others, for example in the study of methane adsorption on activated carbon in the presence of Ni. It can be concluded that nickel on solid surfaces can enhance the adsorption of methane for energy storage.























Similar content being viewed by others
Availability of data and materials
The data presented in this study are available upon request.
References
Allen, M.P., Tildesley, D.J.: Computer simulation of liquids. Oxford University Press, Oxford (1987)
Boonfung, C., Ketprasoet, N., Tangsathitkulchai, C., Wongkoblap, A.: Effects of functional group and surface roughness on adsorption of carbon dioxide in porous glass by grand canonical monte carlo simulation study. J. KMUTNB. (2018). https://doi.org/10.14416/j.kmutnb.2018.01.010
Boonfung, C., Tangsathitkulchai, C., Wongkoblap, A.: Carbon Dioxide capture in homogeneous and heterogeneous surfaces of porous silica glass. Processes. 8, 1260 (2020). https://doi.org/10.3390/pr8101260
Bukhari, S.N., Chi, C.C., Herma, D.S., Nurul, A., Aziz, M.A.A., Aishah, A.J., Sim, Y.C.: Optimal Ni loading towards efficient CH4 production from H2 and CO2 over Ni supported onto fibrous SBA-15. Int. J. Hydrogen Energy 44, 7228–7240 (2019). https://doi.org/10.1016/j.ijhydene.2019.01.259
Calero, S., Dubbeldam, D., Krishna, R., Smit, B., Vlugt, T.J.H., Denayer, J.F.M., Martens, J.A., Maesen, T.L.M.: Understanding the role of sodium during adsorption: a force field for alkanes in sodium-exchanged faujasites. J. Am. Chem. Soc. 126(36), 11377–11386 (2004). https://doi.org/10.1021/ja0476056
Chen, Y., Wu, H., Daofei, L., Yang, W., Qiao, Z., Li, Z., Xia, Q.: An ultramicroporous Nickel-Based Metal-Organic framework for adsorption separation of CO2 over N2 or CH4. Energy Fuels 32, 8676–8682 (2018). https://doi.org/10.1021/acs.energyfuels.8b02287
Egon, W., Nils, W., Holleman, A.F.: Inorganic chemistry. Academic Press; Berlin; New York, San Diego (2001)
Fan, C., Zeng, Y., Do, D.D., Nicholson, D.: A molecular simulation study of adsorption and desorption in closed end slit pores: Is there a hysteresis loop? Chem. Eng. Sci. 121, 313–321 (2015). https://doi.org/10.1016/j.ces.2014.08.018
Ghaemi, A., Hossein, M., Zohourian, I.P.: NiO and MgO/activated carbon as an efficient CO2 adsorbent: characterization, modeling, and optimization. Int. J. Environ. Sci. Technol. 19, 727–746 (2022). https://doi.org/10.1007/s13762-021-03582-x
Guo, J.Z., Hou, Z., Gao, J., Zheng, X.: DRIFTS study on adsorption and activation of CH4 and CO2 over Ni/SiO2 catalyst with various Ni Particle Sizes. Chin. J. Catal. 28, 22–26 (2007). https://doi.org/10.1016/s1872-2067(07)60009-6
Harris, J.G., Yung, K.H.: Carbon dioxide’s Liquid-Vapor coexistence curve and critical properties as predicted by a simple molecular model. J. Phys. Chem. 99, 12021–12024 (1995). https://doi.org/10.1021/j100031a034
He, Y.F., Seaton, N.A.: Experimental and computer simulation studies of the adsorption of ethane, carbon dioxide, and their binary mixtures in MCM-41. Langmuir 19, 10132–10138 (2003). https://doi.org/10.1021/la035047n
Heuchel, M., Davies, G., Buss, E., Seaton, N.A.: Adsorption of carbon dioxide and methane and their mixtures on an activated carbon: simulation and experiment. Langmuir 15, 8695–8705 (1999). https://doi.org/10.1021/la9904298
Hildebrand, F.E., Abeyaratne, R.: An atomistic investigation of the kinetics of detwinning. J. Mech. Phys. Solids 56, 1296–1319 (2008). https://doi.org/10.1016/j.jmps.2007.09.006
Johnson, J.K., Zollweg, J.A., Gubbins, K.E.: The Lennard-Jones equation of state revisited. Mol. Phys. 78, 591–618 (1993). https://doi.org/10.1080/00268979300100411
Kim, J.W., Kim, L.U., Kim, C.K.: Size control of silica nanoparticles and their surface treatment for fabrication of dental nanocomposites. Biomacromol 8, 215–222 (2007). https://doi.org/10.1021/bm060560b
Koh, C.A., Montanari, T., Nooney, R.I., Tahir, S.F., Westacott, R.E.: Experimental and computer simulation studies of the removal of carbon dioxide from mixtures with methane using AlPO4-5 and MCM-41. Langmuir 15, 6043–6049 (1999). https://doi.org/10.1021/la9814337
Kohmuean, P., Inthomya, W., Wongkoblap, A., Tangsathitkulchai, C.: Monte carlo simulation and experimental studies of CO2, CH4 and their mixture capture in porous carbons. Molecules 26, 2413 (2021). https://doi.org/10.3390/molecules26092413
Li, X., Ding, Y., Guo, L., Liao, Q., Zhu, X., Wang, H.: Non-aqueous energy-efficient absorbents for CO2 capture based on porous silica nanospheres impregnated with amine. Energy 171, 109–119 (2019). https://doi.org/10.1016/j.energy.2018.12.175
Liu, H., Ding, W., Lei, S., Tian, X., Zhou, F.: selective adsorption of CH4/N2 on Ni-based MOF/SBA-15 composite materials. Nanomaterials 9, 149–149 (2019). https://doi.org/10.3390/nano9020149
Nascimento do, A.R., de Figueredo, G.P., da Costa, T.R., de Melo, M.A.F., de Melo, D.M.A., de Souza, M.J.B.: Thermodynamics of CO2 adsorption on mesoporous materials impregnated with nickel. Cerâmica 63, 524–529 (2017). https://doi.org/10.1590/0366-69132017633682144
Nicholson, D., Parsonage, N.G.: Computer Simulation and the Statistical Mechanics of Adsorption. Academic Press, Cambridge (1982)
Nimjaroen, C., Morimoto, S., Tangsathitkulchai, C.: Preparation and properties of porous glass using fly ash as a raw material. Journal of Non Crystalline Solids. 355, 1737–1741 (2009). https://doi.org/10.1016/j.jnoncrysol.2009.06.016
Park, J.G., Chang, H.K., Yi, K., Park, J.H., Han, S.S., Cho, S.H., Kim, J.N.: Reactive adsorption of sulfur compounds in diesel on nickel supported on mesoporous silica. Appl. Catalysis B-environmental. 81, 244–250 (2008). https://doi.org/10.1016/j.apcatb.2007.12.014
Peng, X., Zhou, J., Wang, W., Cao, D.: Computer simulation for storage of methane and capture of carbon dioxide in carbon nanoscrolls by expansion of interlayer spacing. Carbon 48, 3760–3768 (2010). https://doi.org/10.1016/j.carbon.2010.06.038
Samios, S., Stubos, A.K., Kanellopoulos, N.K., Cracknell, R.F., Papadopoulos, G.K., Nicholson, D.: Determination of micropore size distribution from Grand Canonical Monte Carlo simulations and experimental CO2 isotherm data. Langmuir 13, 2795–2802 (1997)
Shen, Y., Shi, W., Zhang, D., Na, P., Fu, B.: The removal and capture of CO2 from biogas by vacuum pressure swing process using silica gel. J. CO2 Util. 27, 259–271 (2018). https://doi.org/10.1016/j.jcou.2018.08.001
Sriling, P., Wongkoblap, A., Tangsathitkulchai, C.: Computer simulation study for methane and hydrogen adsorption on activated carbon based catalyst. Adsorption 22, 707–715 (2016). https://doi.org/10.1007/s10450-016-9763-3
Tatsuda, N., Goto, Y., Setoyama, N., Fukushima, Y.: Adsorption of carbon dioxide on mesoporous silicas near the critical temperature. Adsorpt. Sci. Technol. 23, 763–776 (2005). https://doi.org/10.1260/026361705776316569
Teerachawanwong, P., Makkaroon, B., Boonfung, C., Tangsathitkulchai, C., Wongkoblap, A.: Computer simulation study for functional group effect on methane adsorption in porous silica glass. Eng. J. 23, 197–204 (2019). https://doi.org/10.4186/ej.2019.23.5.197
Witoon, T., Chareonpanich, M.: Effect of pore size and surface chemistry of porous silica on CO2 adsorption. Songklanakarin J. Sci. Technol. 34(4), 403–407 (2012)
Wongkoblap, A., Junpirom, S., Do, D.: Adsorption of Lennard-Jones fluids in carbon slit pores of a finite length. A computer simulation study. Adsorption Sci Technol. 23, 1–18 (2004). https://doi.org/10.1260/0263617053737163
Yazawa, T.: Present status and future potential of preparation of porous glass and its applications. Key Eng. Mater. 115, 125–146 (1995). https://doi.org/10.4028/www.scientific.net/kem.115.125
Yue, X., Yang, X.: Molecular simulation study of adsorption and diffusion on silicalite for a Benzene/CO2 mixture. Langmuir 22, 3138–3147 (2006). https://doi.org/10.1021/la052843f
Yun, J.H., Duren, T., Keil, F.J., Seaton, N.A.: Adsorption of methane, ethane, and their binary mixtures on MCM-41: experimental evaluation of methods for the prediction of adsorption equilibrium. Langmuir 18(7), 2693–2701 (2002). https://doi.org/10.1021/la0155855
Zieliński, M., Wojcieszak, R., Monteverdi, S., Mercy, M., Bettahar, M.M.: Hydrogen storage on nickel catalysts supported on amorphous activated carbon. Catalysis Commun. 6, 777–783 (2005). https://doi.org/10.1016/j.catcom.2005.07.001
Acknowledgements
This work was supported by Suranaree University of Technology. We also acknowledge the National e-Science Infrastructure Consortium for providing the computer simulation.
Funding
This research received funding from Suranaree University of Technology in the term of scholarship to Pakamas Kohmuean and Supawan Inthawong.
Author information
Authors and Affiliations
Contributions
Methodology, writing original draft by PK and SI; supervise, writing review and editing by AW. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kohmuean, P., Inthawong, S. & Wongkoblap, A. Nickel effects on carbon dioxide and methane adsorptions on porous glass: experimental and monte carlo simulation studies. Adsorption 30, 313–327 (2024). https://doi.org/10.1007/s10450-023-00421-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-023-00421-y