Abstracts
A series of mixed oxides was synthesized by deposition of the guest phase on the highly dispersed oxide matrix. Fumed nanooxides SiO2, Al2O3, SiO2/Al2O3, and SiO2/Al2O3/TiO2 with the specific surface area of 65–91 m2/g were selected as highly dispersed matrices. Co–Fe mixed oxides with the general formula Co4xFexOy (Co: Fe = 4: 1) were deposited as the guest oxides using the two-step method: (i) solvate-stimulated modification of the surface of fumed nanocarriers with the mixture of cobalt nitrate (II) and iron (III) formate and (ii) subsequent heat treatment up to 600 °C to form Co4xFexOy. The aim of this paper was to study the influence of the composition and structure of fumed oxide matrices and deposited guest phase on the morphology of the resulting composites in the gaseous and aqueous media using the XRD, XPS, FTIR, nitrogen adsorption and SEM/EDX, as well as quasi-elastic light scattering (QELS) methods. The low-temperature nitrogen adsorption isotherms have a sigmoidal shape with a narrow hysteresis loop characteristic of mesoporous materials. The specific surface area (SBET) of the composites varies from 48 to 82 m2/g, showing a tendency towards a decrease in the SBET values by 10–26% in comparison with the initial nanocarriers. The SEM data show the denser aggregate structure of nanocomposites compared to the initial carriers. The primary particle size was in the 30–60 nm range and the EDX data confirm the formation of a guest phase on the mixed aluminosilicate carriers, mainly in the surface patches corresponding to the alumina structure. According to the QELS data, there is a tendency to form aggregates of 100–10 μm in size in the aqueous media. The XRD method shows that the deposited metal oxides are in the form of crystalline phases of Co3O4 with the crystallites of 25–26 nm in size for the individual SiO2 and Al2O3 nanocarriers and 34–37 for the mixed ones, but the iron oxide reflections were not identified for the composites. XPS observation demonstrates the signal of Fe 2p electrons as the form of Fe2O3 oxide in the surface layer of nanocomposites as well as Co 2p as the Co3O4 and Co(OH)2.
Similar content being viewed by others
1 Introduction
Nanotechnology is a topic which is currently being given a lot of attention by researchers because the unique chemical and physical properties of the nanomaterials have led to important roles in many scientific and industrial fields [1,2,3].
In particular, the current trend of the nanomaterials development is the synthesis of new sorbents with a large specific surface area and various active sites [4] for the effective interactions with various pollutant molecules, such as dyes [5,6,7,8], metal ions [9,10,11,12,13], high molecular weight compounds [14, 15]. High-dispersed oxides (SiO2, Al2O3, TiO2) can be used as effective agents in the processes of sorption [16] and flocculation [14, 17, 18] of water-soluble polymers. However, using highly dispersed sorbents in the form of fine powders in the liquid dispersion medium poses the task of their extraction after the sorption process. One of the ways to solve this problem is to create highly dispersed sorbents with additional functionality—magnetically sensitive composite nanomaterials [19, 20]. In most cases, the addition of nanomagnetite [21, 22] to the composition of such materials provides magnetic sensitivity but this has some limitations in the use, for example, high temperature can cause a decrease in magnetic properties [23]. In addition to magnetite, nickel and cobalt ferrites can exhibit magnetosensitivity. Immobilization of these compounds on the surface of highly dispersed oxides provides them with high thermal stability.
Furthermore, their interesting magnetic properties [24,25,26] suggest that they will have many applications in the magneto-optical recording media, displays and devices such as waveguides, insulators and switches [27]. There are different synthetic methods [28, 29] to prepare iron- and cobalt oxide nanoparticles: sol–gel [30], co-precipitation [31], plasma coating [32], sintering [33, 34], hydrothermal synthesis [35], and wetness- or incipient wetness impregnation [25]. Identifying the best combination of physical and chemical parameters for a specific application is a critical and ongoing research process [36]. The control over these parameters in metal oxide NPs for many applications is achieved by modifying the traditional synthetic procedures (following precipitation or high-temperature decomposition pathways) such as varying the temperature, the ratio of reactants, used surfactants, pH, or solvent [37, 38]. However, it remains a challenge to obtain ultrafine NPs with high stability and narrow size distribution. To overcome these limitations, a suitable and cost-effective strategy is grafting transition metal oxides on the surface of an appropriate material, in particular, highly dispersed supports. It has been reported that Co–Fe oxide nanoparticles were fabricated on various supports, including silica [27], alumina [39], zirconia [40] and Titania [25]. Among them, fumed silica dioxide (SiO2) is highly mechanical and chemical stable, macroporous structure, has a large specific area, is environmentally friendly, and less toxic in nature [41,42,43]. For instance, the authors [44] obtained iron-cobalt nanoparticles dispersed in silica matrix and noted the formation of magnetic cobalt ferrite nanocomposites, Co3O4 and ferrihydrite or Co–Fe alloys, depending on the Fe and Co precursors. The previously synthesized nickel–cobalt oxide nanoparticles deposited on the fumed silica have proved themselves as promising catalysts for CO2 methanation [45].
The synthesis of nanocomposites (NCs) based on the highly dispersed carriers with deposited metal oxides allows directing the design of a potential adsorbent towards increasing sorption properties and providing multifunctionality [46]. The introduction of Co–Fe nanoparticles into the structure of composites requires a study of the influence of synthesis conditions and the type of substrate on the structure and properties of NCs in general and the magnetosensitive phase in particular. The use of different carriers with different surface structures can affect significantly the structure of the deposited phase [47]. Moreover, there are no reports related to NCs based on the transition metals Co–Fe, supported on highly dispersed mixed nanooxides such as SiO2/Al2O3 and SiO2/Al2O3/TiO2 with detailed investigations of their surface compositions. The presence of support provides new means to tailor the structural performance by altering the exposure of the active sites and modifying their nature through the interaction of the transition metal oxides with the support.
In the light of the above, the present paper was undertaken with the aim of analysing the influence of carries such as fumed silica, alumina, silica/alumina, and alumina/silica/titania on the formation of the grafted mixed oxide Co4xFexOy as well as the homogeneity, morphology and structure of the NCs as a whole.
2 Materials and methods
2.1 Materials
Fumed oxides SiO2 (A-60), Al2O3, SiO2/Al2O3 (SA96), and Al2O3/SiO2/TiO2 (AST1) (Pilot plant of Chuiko Institute of Surface Chemistry, Kalush, Ukraine) with similar values of the specific surface area (SBET) of 65–91 m2/g (Table 1) were used as nanocarriers. Fumed oxides are composed of spherical-like nonporous nanoparticles, whose diameter can be estimated as d ≈ 6/(ρ0SBET), where ρ0 is the true density of nonporous nanoparticles. Cobalt nitrate hexahydrate (Co(NO3)2·6H2O, ≥ 98.5%, Merck) and iron (III) formate (Fe(CHO2)3·2H2O, 98%, Merck) were used as precursors to synthesize the nanooxides composites.
2.2 Synthesis of Co4xFexOy—containing composites
Co4xFexOy/nanocarrier composites (Table 1) were prepared using a two-stage method. The first stage was the solvate-stimulated modification of the surface of the initial fumed oxides with the mixture of Co(II) nitrate and Fe(III) formate in the ceramic ball-mill. The second stage consisted of drying and subsequent heating of the resulting mixture at 600 °C. The pre-modification of a fumed nanooxide carrier with the concentrated solutions of salts of the corresponding metals was performed in the 800 cm3 ceramic ball-mill (ball size 2–3 cm, speed of 60 rpm) for 1.5 h. The mill was charged with 20 g of fumed oxide carrier and 10 ml of the aqueous solution of Co(II) nitrate (34.8 mmol) and Fe(III) formate (9.4 mmol) weighed to obtain a content in the deposits in the resulting composite. After achieving homogeneity, according to the microscopic examination, the resulting mixture was air-dried for 24 h. The dried powder was calcined at 600 °C for 1 h. The feature of the solvate-stimulated modification method is the homogeneous distribution of the solvated salt over the carrier surface. This is a required condition to form nanosized crystallites of the deposited oxide phase by the salt thermolysis upon the calcination at 600 °C.
2.3 Textural characterization
To analyse the textural characteristics of Co4xFexOy/fumed oxide NCs, low-temperature (77.4 K) nitrogen adsorption–desorption isotherms were recorded using a Micromeritics ASAP 2405N adsorption analyzer. The samples were degassed at 110 °C for 2 h in the vacuum chamber. The values of the specific surface area (SSA, SBET) were calculated according to the standard BET method [48]. The total pore volume Vp was evaluated by converting the volume of adsorbed nitrogen at p/p0 = 0.98–0.99 (p and p0 denote the equilibrium and saturation pressures of nitrogen at 77.4 K, respectively) to the volume of liquid nitrogen per gram of adsorbent. The nitrogen desorption data were used to compute the pore size distributions (PSDS, differential fV–dVp/dR and fS–dS/dR) using a self-consistent regularization (SCR) procedure under the non-negativity condition (fV ≥ 0 at any pore radius R) at a fixed regularization parameter α = 0.01 with voids (V) between spherical nonporous nanoparticles packed in random aggregates (V/SCR model) [49]. The differential PSDS with respect to the pore volume fV ~ dV/dR, ∫fVdR ~ Vp were re-calculated to incremental PSD (IPSD) at ΦV(Ri) = (fV(Ri+1) + fV(Ri))(Ri+1 − Ri)/2 at ∑ΦV(Ri) = Vp. The fV and fS functions were also used to calculate contributions of nanopores (Vnano and Snano at 0.35 < R < 1 nm), mesopores (Vmeso and Smeso at 1 < R < 25 nm), and macropores (Vmacro and Smacro at 25 < R < 100 nm).
2.4 Scanning electron microscopy (SEM)
The surface morphology of composites was analyzed using the field emission Scanning Electron Microscopy employing a QuantaTM 3D FEG (FEI, USA) apparatus operating at the voltage of 30 kV.
2.5 X-ray powder diffraction
X-ray powder diffraction (XRD) patterns of metal oxide (as-synthesized) and metal NCs (after testing in the catalytic process) were recorded on the DRON-3 M diffractometer (Burevestnik, St.-Petersburg, Russia) with the monochromatic CuKα radiation (λ = 0.15418 nm) and the Ni filter over the angular range from 5° to 90°. The X-ray tube operation parameters were: voltage of 40 kV, current of 15 mA, scanning speed 2° min–1. Interpretation of the phase composition was performed using the analytical software PDXL-2. The crystalline structure of the samples was analyzed using the JCPDS Database [50]. The average sizes of nanocrystallites (d, nm) were estimated according to the Scherrer equation [51] the calculations took into account the internal standard of the device (α-quartz)
where d is the dimension of the particle in the direction perpendicular to the reflecting plane, λ is the X-ray wavelength, K is the particle shape coefficient (equal to 0.9), L is the width of the reflex at half of its height, and θ is the scattering angle.
2.6 Fourier transform infrared (FTIR) spectroscopy
FTIR spectra of the powdered samples were recorded over the 4000–400 cm−1 range using the IR Spirit FTIR with the ATR mode.
2.7 X-ray photoelectron spectroscopy (XPS)
X-ray photoelectron spectroscopy (XPS) studies were performed using the multi-chamber UHV system (PREVAC). Spectra were collected using hemispherical Scienta R4000 electron analyser. Scienta SAX-100 x-ray source (Al Kα, 1486.6 eV, 0.8 eV band) equipped with the XM 650 X-Ray Monochromator (0.2 eV band) were used as a complementary equipment.
The pass energy of the analyser was set to 200 eV for survey spectra (with 750 meV step), and 50 eV for regions (high resolution spectra): C1s, O1s and N1s with 50 meV step). The base pressure in the analysis chamber was 5 × 10–9 mbar. During the spectra collection it was not higher than 3 × 10–8 mbar.
2.8 Particle size distribution in the aqueous media
Particle size distributions (PaSD) of NCs in the aqueous medium were studied with the Zetasizer Nano ZS (Malvern Instruments) apparatus using the universal dip cell (ZEN1002) (Malvern Instruments) and the wavelength λ = 633 nm and as well as the scattering angle of 13 and 176° at 298 K. The particle size determination accuracy and repeatability were ± 1–2%. The refractive index and absorption were 1.59 and 0.01, respectively. The samples for the PaSD measurement were prepared as follows: 0.1 wt% suspension of a composite in distilled water was sonicated for 3 min at 22 kHz using the ultrasonic disperser UZDN-A (500 W). The suspensions were equilibrated for 24 h and the PaSD were measured. The real information obtained from the experiment on the quasi-elastic light scattering is the distribution of light scattering intensity (PaDSI) from the objects by diffuse broadening. However, it is not the intensity distribution itself that is of interest but the associated particle size distribution related to the number (PaDSN), or the molecular weight distribution (PaDSM), or the related volume (PaDSV). The collation of this information allows us to describe comprehensively the structure of NC suspensions. The transition from (PaDSI) to these familiar characteristics of a polydisperse system requires the involvement of model ideas about the structure of the diffusers. To perform the measurements there was used, the computer program of the Malvern Instruments Company, which assumes that the particles are spherical.
2.9 Magnetic properties
Magnetic characterization of Co4xFexOy/nanocarrier composites was made in the Quantum Design MPMS SQUID-VSM Evercool Magnetometer (San Diego, USA). The hysteresis loop (at 300 K and 5 K) was recorded with the applied magnetic fields between − 50 and 50 kOe. The saturation magnetisation for each sample was normalised by taking into account the metals content measured by the SEM/EDX analysis. The zero-field-cooled (ZFC) magnetization measurements were made after cooling the samples in the zero field to 5 K and measuring the magnetization while heating the samples up to 300 K in the magnetic field (H = 100 Oe). The field-cooled (FC) curve is measured by changing the temperature from 300 to 5 K.
3 Results and discussion
3.1 Textural characterization
The shape of the nitrogen adsorption–desorption isotherms (Fig. 1) can be attributed to type II with the hysteresis loop H3 of the IUPAC classification [48, 52] indicating the textural porosity of aggregates of nanoparticles. The presence of such a form of hysteresis loop indicates the dominant contribution of mesopores that are filled by nitrogen during the measurements.
The textural characteristics of oxide NCs calculated from the nitrogen adsorption isotherms are given in Table 2. The BET surface area and pore volume of composites (Table 2) depend on the specific surface area (SSA) values of the fumed oxide carriers. In general, after the modification of fumed oxides with Co–Fe mixed oxide, the SSA value of the composite decreases slightly for all carriers. The total pore volume and that of mesopores are typically greater for the composites than for the initial fumed oxides due to the compaction of the powders under the synthesis. The compaction results in a decrease in the empty volume (Vem = 1/ρb − 1/ρ0, where ρb and ρ0 denote the bulk and true densities of the powder materials) in the powders since the bulk density increases. However, the volume of mesopores can be increased due to the closer location of nonporous nanoparticles in their aggregates and agglomerates of aggregates [53]. All synthesized composites demonstrate pore size distributions with a predominant contribution from mesopores (Table 2, Fig. 2), which allows their classification as mesoporous materials.
The pore size distribution functions (Fig. 2) confirm the conclusion based on the isotherm shapes (Fig. 1) that the composites are mainly mesoporous since the contributions of micropores and macropores are small (Table 2). The first peak is due to the really small contribution of nanopores at R < 1 nm. However, the second one is due to the ineffective adsorption of nitrogen in the macropores because the interactions of nitrogen molecules with large distant nanoparticles are very weak. Therefore, nitrogen molecules in the bound fluid are not predominant in broad macropores. The first peak of the PSD (Fig. 2) corresponds to the narrow voids between the nanoparticles closely located in the same aggregates. Broader voids can be present between the distant nanoparticles in the same aggregate or neighbouring aggregates.
The contribution of macropores increases significantly (Fig. 2) for all samples Co4xFexOy/nanocarrier, except for Co4xFexOy/SiO2, for which it remains almost unchanged. The total pore volume Vp and the volume of mesopores increase for all composites compared to the initial fumed oxides (Table 2). As a result, the changes in the pore volume depend on the type of support, features of the second phase formation, decrease in the void volume in the aggregates of nanoparticles, and the difference in the true density of the deposited oxide particles and fumed oxide carriers that is minimal for silica.
3.2 Scanning electron microscopy
The SEM images of Co4xFexOy/fumed oxide NCs (Figs. 3 and 4) show the formation of Co4xFexOy particles at the nanocarrier surface. As shown in Figs. 3 and 4, it can be observed that the Co4xFexOy nanoparticles (NPs) appear in the uniform sphere-like shape and homogeneous distribution. The aggregated structures of grafted oxides of 20 to 40 nm in size were well observed for Co4xFexOy/SiO2, Co4xFexOy/Al2O3 (Fig. 3b, d), Co4xFexOy/SA96 (Fig. 4b) and 80–120 nm for Co4xFexOy/AST1 (Fig. 4d). As a whole, the composites look more compact than the initial fumed nanocarriers.
According to the SEM/EDX data (Table 3), it can be seen that in the case of mixed fumed oxides as carriers, the surface content of aluminium is significantly reduced. This indicates that the synthesis of the deposited oxide phase occurs mainly in the areas corresponding to the Al2O3 patches: for the Al2O3-based composite the decrease in the Al surface concentration was from 50.8 to 34.5%, for the SA96-based composite it was from 48.1 up to 33.6%, for the AST1-based composite it was from 41.8 up to 27.4%.
The surface concentration of Ti atoms also decreases for the AST1-based NCs more than doubled, indicating that the patches of the surface having TiO2 or the solid solution structure with the bridge-type Ti–O–Si or Al–O–Ti sites also take part in the interactions with the deposited phase. At the same time, the Si concentration increases in the composites based on the mixed SA96 and AST1 nanocarriers which indicate that the regions with a structure corresponding to SiO2 remain not involved in the interactions with the deposited phase. The Co and Fe surface concentrations depend insignificantly on the carrier nature and it varies for Co from 14.9 to 15.0 over the individual carriers (Al2O3 and SiO2) and 14.4–14.5 over the mixed carriers (SA96 and AST1) and 3.4–4.1 for Fe. The average Co/Fe wt% ratio calculated from the SEM/EDX data for all NCs is very close to the given values upon the component ratio in the synthesis (Co: Fe = 4: 1).
3.3 X-ray powder diffraction analysis
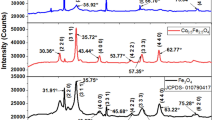
The XRD patterns of Co4xFexOy/fumed oxide NCs (Fig. 5) showed only a cubic cobalt oxide Co3O4 phase formed on the surface of carriers. The diffraction peaks at 2θ = 19.0; 31.2; 36.8; 44.63; 58.98; 65.1 correspond to the reflections (111), (202), (311), (400), (511), (404) of Co3O4. The size of the cobalt oxide crystallites calculated from the plane (311) according to the Scherrer equation is 25–37 nm (Table 4). Smaller crystals are formed on the surface of individual oxides, and the surface of mixed oxides promotes the formation of larger particles. The iron, which was introduced into the composition of NCs by the method of solvate-stimulated surface modification of highly dispersed oxides, is not identified, as an individual phase by the XRD method. The registration of iron compound is complicated due to its low content in the NCs composition and can be under the XRD device sensitivity.
3.4 IR spectroscopy
The IR spectra of the synthesized Co4xFexOy/fumed oxide NCs were characterized using the FTIR spectroscopy in the wavenumber range 4000–400 cm−1 and are shown in Fig. 5. Comparing the IR spectra of initial fumed oxides used for the synthesis with the spectra of synthesized Co4xFexOy/fumed oxide NCs (Fig. 6), it should be noted that in all spectra characteristic bands are observed in the ranges: the broadband between 3700 and 3100 cm−1 refers to the stretching vibrations of OH groups sourced from various forms of adsorbed water. The band at 1636 cm−1 corresponds to the deformation vibrations of water molecules. The broad intensive stretching vibrations of Si–O–Si at 1100 cm−1 (asymmetric) and 800 cm−1 (symmetric) are observed in the silica-based composites.
To characterize the deposited Co4xFexOy phase, the initial IR spectra were processed mathematically: the spectrum of the initial nanocarrier was subtracted from the spectrum of the Co4xFexOy/fumed oxide NCs and the remaining spectrum was decomposed using the Origin7.5 PeakFit, which made it possible to estimate the presence and characteristic frequency of peaks assigned to the Co–O and Fe–O bonds in different sites (Table 5).
The bands in the region of 300–700 cm−1 are assigned to the fundamental vibrations of the metal (M) ions of the crystal lattice in the octahedral and tetrahedral positions [54]. The peak at 652–664 cm−1 corresponds to the intrinsic stretching vibrations of the cobalt in the tetrahedral site, Co2+–O [55] in the tetrahedral position, and 551–567 cm−1 are assigned to Co3+–O in the octahedral position. The absorption band at 525–623 cm−1 can be attributed to the stretching vibrations of Fe–O in the tetrahedral and octahedral sites. Based on the literature data [56, 57] the high-frequency bands about (600–700 cm−1) are attributed to the tetrahedral group, and the low-frequency band (about 350–450 cm−1) is attributed to the octahedral group, Fe in the obtained composites is more likely to belong to the tetrahedral sites while the formation of the structure of the solid solution.
3.5 X-ray photoelectron spectroscopy
The surface analysis of Co–Fe oxides NCs was performed using the XPS method. The survey spectra indicate the presence of O, Fe and Co at the surface of all NCs as well as Al and/or Si at the surface of NCs based on the appropriate carrier. Figures 7 and 8 show typical core-level spectra of Co 2p3/2 and Fe 2p3/2 for synthesized NCs respectively. Experimental Eb values of Co 2p3/2 and Fe 2p3/2 core-level XPS spectra of investigated Co–Fe nanocomposites are presented in Table 6. The XPS peak areas were used for the calculation of Co/Fe and Co3O4/Co(OH)2 ratios.
Fitting of Co 2p3/2 XPS spectra was provided according to the model proposed by Biesinger [58]. XPS signal of Co 2p electrons can be convoluted into two groups of multiple peaks for all of the investigated NCs. The multiple peaks depicted as 1–4 (Fig. 7a–d) with the binding energy of 2p3/2 electrons within the range of 776.7–789.6 eV and multiplet of A–D in the range of 780.5–790.5 eV corresponded to Co3O4 and Co(OH)2 species respectively. The calculated data of atomic % concentrations of corresponding types specified the high level of Co(OH)2 on the surface of NCs. There are two possible reasons for the Co(OH)2 signal on the surface of NCs. The first suggestion is not entirely decomposition of Co(NO3)2·6H2O modifying salts with formation of stable half-product means Co(OH)2 and other one is the secondary hydroxylation of Co2+ surface particles. We declare that the synthesis conditions ensure the entire destruction of grafted modifying nitrate groups with the resulting formation of Co3O4. This is confirmed with XRD results showed the presence Co3O4 compounds in the structure on synthesized NCs. Therefore, the presence of cobalt hydroxide on the surface of NCs can occur as a result of hydroxylation of the surface of NCs. The important observation is concerning of Co3O4/Co(OH)2 ratio for the different NCs. It is noted that the calculated amount of Co3O4 in the surface layer of SiO2 based NC is higher than for other carriers. It seems that amorphous SiO2 is more appropriate for Co3O4 formation in the surface layer of NCs than crystallinity carrier based on the Al2O3 (Al2O3, SA96, AST1). The calculated Co/Fe ratio in the surface layer of Co–Fe NCs (see Table 6) does not correspond to specified in the synthesis routine as Co:Fe = 4:1. It follows that approximately 25% of grafted cobalt compounds are presented on the surface layer and significant part of cobalt can be localized in the pores of NCs. XPS research results show the presence of Fe(III) in the surface layer of nanocomposites [58,59,60]. The most possible compound of Fe (III) that can be formed on the surface of NCs is Fe2O3 [60]. Moreover, the chemical transformation of iron containing surface modification agent (iron formate) leads to the formation of Fe2O3 under the presented synthesis condition. The formation of Fe3O4 is doubtful because it requires either significantly higher temperatures or a reducing atmosphere.
The XPS signal of O 1 s electrons was de-convoluted into several peaks (Fig. 8) related to the carrier matrix and CoFe modified oxides. Experimental Eb values obtained from O 1 s XPS spectra and atomic % calculated as the integrated square of the de-convoluted peaks are provided in Table 7. The main components of oxygen bonds have been identified and related to the highly dispersed matrices of NCs. XPS signals of O 1 s electrons with Eb at 532.7 and 533.3 ± 0.1 eV can be associated with Si–O and Si–OH moieties presented on the surface of SiO2 and AST1-based NCs. For the NCs based on Al2O3, SA96 and AST1 appropriate O 1 s signals with Eb at 531.7 and 532.8 ± 0.1 eV related with Al–O and Al–OH moieties [61]. The presence of physisorbed water was identified as a peak of very low intensity located at the region of higher binding energies around 534 eV (Table 7). The XPS signals of O 1 s electrons corresponded to O2− and –OH moieties are positioned at ~ 529.8 and ~ 530.7 eV respectively for the Co–Fe/Al2O3, Co–Fe/SA96 and Co–Fe/AST1 NCs and shifted to the 530.2 and 531.3 eV for Co–Fe/SiO2. These experimental XPS observations suggest that O2− particles can be attributed to fragments of metal oxides (Co and Fe) deposited on the surface of the carrier, and HO– particles are associated with Co(OH)2 groups found on the surface of NCs (Fig. 9).
3.6 Particle size distribution in the aqueous medium
The quasi-elastic light scattering (QELS) method allows determining the particle size distribution (PaSD) in the aqueous medium. The results of measurements for the synthesized samples of mixed oxides are presented in the form of the dependence of the particle size distribution for their number PaSDN (Fig. 10a, c) and volume PaSDV (Fig. 10b, d). The modification of silica with Co and Fe oxides leads to an increase in the particle sizes due to the formation of the second oxide phase and enhanced aggregation during the synthesis. The degree of aggregation/agglomeration depends on the characteristics of nanoparticles and their interactions in the dispersion media. The initial fumed oxides (SiO2, Al2O3, SA96) are characterized by nearly monomodal PaSD related to the particle numbers with the maximum at 100–110 nm (Fig. 10), except for AST1 having more complicated particulate morphology.
The PaSDN of the composites are mono- or bimodal (Fig. 10a, c) and three peaks are observed for PaSDV (Fig. 10b, d): aggregates ~ 90 and ~ 430 nm (SiO2) and large agglomerates > 5.5 μm are presented in the aqueous dispersion of composites after the ultrasonic treatment. It should be noted that although for the composites PaSDN shows the maximum at 85–103 nm for SiO2 and the SA96 carrier, i.e., the number of small secondary particles of ~ 100 nm is larger than the number that of large aggregates (> 1 μm). However, according to PaSDV, the volume of aggregates > 1 μm for these composites is much higher than that for the initial nanocarriers. The PaSD for the Co- and Fe-containing composites supported on Al2O3 and AST1 is characterized by the presence of aggregates and agglomerates of larger sizes (~ 1.1–1.3 and 5.5 μm). The aggregates smaller than 1 μm in size are not observed in the dispersion. This indicates stronger bonds of the deposited guest oxide with the support.
3.7 Magnetic properties
The magnetic properties of Co4xFexOy/fumed oxide NCs and their magnetization curves are shown in Table 8 and Fig. 11 of the samples. The hysteresis loop with the superparamagnetic behaviour for Co4xFexOy/SA96 and Co4xFexOy/A2O3 (Hc = 43.05 Oe and 56.5 Oe respectively) and the mix of superpara- and ferromagnetic for the Co4xFexOy/Si60 and Co4xFexOy/AST1 nanocomposites (Hc = 386.5 Oe, and 117.8 Oe, respectively) are pointed out to. The influence of the nonmagnetic phase (nanocarriers) was manifested in the relatively small total magnetization of the synthesized NCs. Apparently, the magnetic properties of the samples Co4xFexOy/SiO2, Co4xFexOy/Al2O3, Co4xFexOy/SA96 and Co4xFexOy/AST1 are influenced by the fraction of pores and their distribution in the composites. Porosity leads to decreased density, impeding magnetic domain rotation in the direction of the magnetic field thus reducing the ability of magnetic conductivity [62, 63]. Pores act as air gaps in the magnetic circuit and decrease the permeability of the material.
The ZFC and FC (Fig. 12) curves at 100 Oe reveal that the blocking temperature for the Co4xFexOy/fumed oxide NCs with the crystallite size around 25–37 nm (Table 8) is much above room temperature (300 K) for all NCs. The studied samples show a mixture of superpara- and ferromagnetic behaviour below the blocking temperature i.e., 300 K. The relatively larger blocking temperature might be due to the large anisotropy in Co4xFexOy phase.
4 Conclusions
The series of novel Co4xFexOy/fumed oxide nanocomposites were synthesized by the thermooxidation of metal salts adsorbed onto the individual and complex fumed nanooxides. The effects of the support structure were analyzed with respect to the formation of the deposited phases. The phase composition and average sizes of crystallites determined from the XRD data show that the Co3O4 phase in the Co4xFexOy/fumed oxide nanocomposites includes crystallites of 25–37 nm in diameter while the iron oxide reflections are not practically identified. The results of XPS measurements are in the good agreement with the FTIR spectroscopy: these methods demonstrated the presence of Co (II) and Co (III) compounds on the surface of nanocomposites as well as XRD result demonstrated formation the crystallites of Co3O4 that can be formed in the porous structure of nanocomposites. According to the SEM/EDX data, the formation of the deposited Co4xFexOy phase occurs mainly on the nanocarrier surface corresponding to the Al2O3 structure patches or solid solution structures with the bridge bonds Al–O–Ti, Al–O–Si and Si–O–Ti. The specific surface area of the composites decreases insignificantly when the phase Co4xFexOy is deposited on the support. The incremental pore size distribution functions show predominantly the mesoporosity of nanocomposites, the volume of mesopores increases significantly in comparison to the initial fumed oxides. According to the QELS data for the aqueous dispersions of the synthesized nanocomposites, small aggregates with several nanoparticles of 100 nm in size, large aggregates up to ~ 1 μm, and agglomerates up to 5.5 μm are observed. There is a general tendency to increase the sizes of aggregates due to the modification of nanocarriers with the deposited Co4xFexOy. This paper demonstrated the effects of various highly dispersed matrices on the magnetic properties of composites. Magnetic properties of the samples Co4xFexOy/SiO2, Co4xFexOy/Al2O3, Co4xFexOy/SA96 and Co4xFexOy/AST1 are influenced by the fraction of pores and their distribution in the composites. Increasing the magnetic phase in the nanocomposites can provide promising adsorbents for the future application.
Data availability
The data will be available at the e-mail address terpil@umcs.pl.
References
Havancsák, K.: Nanotechnology at present and its promise for the future. Mater. Sci. Forum 414, 85–94 (2003). https://doi.org/10.4028/www.scientific.net/MSF.414-415.85
Mohseni, E., Mendizadeh, M.B., Jian, Y., Ali, Y.M.: Single and combined effects of nano-SiO2, nano-Al2O3 and nano-TiO2 on the mechanical, rheological and durability properties of self-compacting mortar containing fly ash. Constr. Build. Mater. 84, 331–340 (2015). https://doi.org/10.1016/j.conbuildmat.2015.03.006
Tamirat, Y.: The role of nanotechnology in semiconductor industry: review article. J. Mater. Sci. Nanotechnol. 5, 202–229 (2017). https://doi.org/10.15744/2348-9812.5.202
Gun’ko, V.M., Leboda, R., Skubiszewska-Zięba, J.: Heating effects on morphological and textural characteristics of individual and composite nanooxides. Adsorption 15, 89–98 (2009). https://doi.org/10.1007/s10450-009-9160-2
Chibowski, S., Wiśniewska, M., Wawrzkiewicz, M., Hubicki, Z., Goncharuk, O.: Electrokinetic properties of silica-titania mixed oxide particles dispersed in aqueous solution of C.I. Direct Yellow 142 dye—effects of surfactant and electrolyte presence. Physicochem. Probl. Miner. Process. 56, 6–13 (2020). https://doi.org/10.37190/ppmp/123612
Chowdhury, A.-N., Rahim, A., Ferdosi, Y.J., Azam, M.S., Hossain, M.M.: Cobalt–nickel mixed oxide surface: a promising adsorbent for the removal of PR dye from water. Appl. Surf. Sci. 256, 3718–3724 (2010). https://doi.org/10.1016/j.apsusc.2010.01.013
Wawrzkiewicz, M., Wiśniewska, M., Gun’ko, V.M., Zarko, V.I.: Adsorptive removal of acid, reactive and direct dyes from aqueous solutions and wastewater using mixed silica–alumina oxide. Powder Technol. 278, 306–315 (2015). https://doi.org/10.1016/j.powtec.2015.03.035
Wiśniewska, M., Wrzesińska, K., Wawrzkiewicz, M., Chibowski, S., Urban, T., Goncharuk, O., Gun’ko, V.M.: Alumina-silica-titania adsorbent for hazardous azo and phthalocyanine dyes removal from textile baths and wastewaters—the impact of ionic surfactants. Physicochem. Prob. Miner. Process. 56, 178–193 (2020). https://doi.org/10.37190/ppmp/128214
Borai, E.H., El-Dessouky, S.I., Hassan, H.S.: Mixed silica and alumina hosted carboxylate oxide for removal of chromium species from wastewater. Adsorption 13, 61–71 (2007). https://doi.org/10.1007/s10450-007-9001-0
Ciesielczyk, F., Bartczak, P., Wieszczycka, K., Siwińska-Stefańska, K., Nowacka, M., Jesionowski, T.: Adsorption of Ni(II) from model solutions using co-precipitated inorganic oxides. Adsorption 19, 423–434 (2013). https://doi.org/10.1007/s10450-012-9464-5
Heredia, A., Gómez Avila, J., Vinuesa, A., Saux, C., Mendoza, S.M., Garay, F., Crivello, M.: Compared arsenic removal from aqueous solutions by synthetic mixed oxides and modified natural zeolites. Adsorption 25, 1425–1436 (2019). https://doi.org/10.1007/s10450-019-00109-2
Ismail, A.A., El-Midany, A.A., Ibrahim, I.A., Matsunaga, H.: Heavy metal removal using SiO2–TiO2 binary oxide: experimental design approach. Adsorption 14, 21–29 (2008). https://doi.org/10.1007/s10450-007-9042-4
Rudziński, W., Charmas, R.: Effects of surface heterogeneity of oxides in simple ion adsorption at oxide/electrolyte interfaces. Adsorption 2, 245–255 (1996). https://doi.org/10.1007/BF00128306
Wiśniewska, M., Bogatyrov, V., Ostolska, I., Szewczuk-Karpisz, K., Terpiłowski, K., Nosal-Wiercińska, A.: Impact of poly(vinyl alcohol) adsorption on the surface characteristics of mixed oxide MnxOy–SiO2. Adsorption 22, 417–423 (2016). https://doi.org/10.1007/s10450-015-9696-2
Wiśniewska, M., Nowicki, P., Bogatyrov, V.M., Nosal-Wiercińska, A., Pietrzak, R.: Comparison of adsorption properties of MgxOy–SiO2 and ZnxOy–SiO2 in the mixed oxide-poly(vinyl alcohol) system. Colloids Surf A Physicochem Eng Asp 492, 12–18 (2016). https://doi.org/10.1016/j.colsurfa.2015.12.013
Wiśniewska, M., Wawrzkiewicz, M., Wołowicz, A., Goncharuk, O.: Nanosized oxides of different compositions as adsorbents for hazardous substances removal from aqueous solutions and wastewaters. In: Fesenko, O., Yatsenko, L. (eds.) Nanooptics, nanophotonics, nanostructures, and their applications, pp. 103–126. Springer, Cham (2018)
Wiśniewska, M., Urban, T., Grządka, E., Zarko, V.I., Gun’ko, V.M.: Comparison of adsorption affinity of polyacrylic acid for surfaces of mixed silica–alumina. Colloid Polym. Sci. 292, 699–705 (2014). https://doi.org/10.1007/s00396-013-3103-x
Wiśniewska, M., Ostolska, I., Szewczuk-Karpisz, K., Chibowski, S., Terpiłowski, K., Gun’ko, V.M., Zarko, V.I.: Investigation of the polyvinyl alcohol stabilization mechanism and adsorption properties on the surface of ternary mixed nanooxide AST 50 (Al2O3–SiO2–TiO2). J. Nanopart. Res. 17, 12–26 (2015). https://doi.org/10.1007/s11051-014-2831-2
Behrens, S., Appel, I.: Magnetic nanocomposites. Curr. Opin. Biotechnol. 39, 89–96 (2016). https://doi.org/10.1016/j.copbio.2016.02.005
Mourdikoudis, S., Kostopoulou, A., LaGrow, A.P.: Magnetic nanoparticle composites: synergistic effects and applications. Adv. Sci. 8, 2004951 (2021). https://doi.org/10.1002/advs.202004951
Giraldo, L., Erto, A., Moreno-Piraján, J.C.: Magnetite nanoparticles for removal of heavy metals from aqueous solutions: synthesis and characterization. Adsorption 19, 465–474 (2013). https://doi.org/10.1007/s10450-012-9468-1
Fadillah, G., Yudha, S.P., Sagadevan, S., Fatimah, I., Muraza, O.: Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications. Open Chem. 18, 1148–1166 (2020). https://doi.org/10.1515/chem-2020-0159
Ramirez, S., Chan, K., Hernandez, R., Recinos, E., Hernandez, E., Salgado, R., Khitun, A.G., Garay, J.E., Balandin, A.A.: Thermal and magnetic properties of nanostructured ferrimagnetic composites with graphene–graphite fillers. Mater. Des. 118, 75–80 (2016). https://doi.org/10.1016/j.matdes.2017.01.018
Ali, A., Zafar, H., Zia, M., Ihsan Ul, H., Rehman, P.A., Ali, J.S., Hussain, A.: Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 9, 49–67 (2016). https://doi.org/10.2147/NSA.S99986
Duvenhage, D.J., Coville, N.J.: Fe:Co/TiO2 bimetallic catalysts for the Fischer–Tropsch reaction: part 3: the effect of Fe: Co ratio, mixing and loading on FT product selectivity. Appl. Catal. A Gen. 289, 231–239 (2005). https://doi.org/10.1016/j.apcata.2005.05.008
Matzui, LYu., Vovchenko, L.L., Syvolozhskyi, O.A., Yakovenko, O.S., Borovoy, M.O., Gomon, O.O., Dyachenko, A.G., Ischenko, O.V., Vakaliuk, A.V., Bodnaruk, A.V., Kalita, V.M.: Structure and magnetic properties of MWCNTs decorated by NiFe, CoFe, NiCo nanoparticles. Mol. Cryst. Liq. Cryst. 752, 77–94 (2023). https://doi.org/10.1080/15421406.2022.2091275
O’Shea, V.A., Álvarez-Galván, M.C., Campos-Martin, J.M., Menéndez, N.N., Tornero, J.D., Fierro, J.L.G.: Surface and structural features of Co–Fe oxide nanoparticles deposited on a silica substrate. Eur. J. Inorg. Chem. 24, 5057–5068 (2006). https://doi.org/10.1002/ejic.200600778
Yathindranath, V., Worden, M., Sun, Z., Miller, D.W., Hegmann, T.: A general synthesis of metal (Mn, Fe Co, Ni, Cu, Zn) oxide and silica nanoparticles based on a low temperature reduction/hydrolysis pathway. RSC Adv. 3, 23722–23729 (2013). https://doi.org/10.1039/c3ra44565h
Zhludenko, M., Dyachenko, A., Bieda, O., Gaidai, S., Filonenko, M., Ischenko, O.: Structure and catalytic properties of Co–Fe systems in the reaction of CO2 methanation. Acta Phys. Polonica A 133, 1084–1087 (2018). https://doi.org/10.12693/APhysPolA.133.1084
Huang, X., Chen, Z.: Sol–gel preparation and characterization of CoFe2O4–SiO2 nanocomposites. Solid State Commun. 132, 845–850 (2004). https://doi.org/10.1016/j.ssc.2004.09.060
Teoh, L.G., Li, K.-D.: Synthesis and characterization of NiO nanoparticles by sol–gel method. Mater. Trans. 53, 2135–2140 (2012). https://doi.org/10.2320/matertrans.M2012244
Dalai, A.K., Bakhshi, N.N., Esmail, M.N.: Characterization studies of plasma-sprayed cobalt and iron catalysts. Ind. Eng. Chem. Res. 31, 1449–1457 (1992). https://doi.org/10.1021/ie00006a005
Borysenko, M.V., Gun’ko, V.M., Dyachenko, A.G., Sulim, I.Y., Leboda, R., Skubiszewska-Zieba, J., Ryczkowski, J.: CVD-zirconia on fumed silica and silica gel. Appl. Surf. Sci. 242, 1–12 (2005). https://doi.org/10.1016/j.apsusc.2004.07.064
Dry, M.E.: The Fischer–Tropsch synthesis. In: Anderson, J.R., Boudart, M. (eds.) Catalyst science technology, pp. 159–255. Springer-Verlag, Berlin (1981)
Beach, E.R., Shqau, K., Brown, S.E., Rozeveld, S.J., Morris, P.A.: Solvothermal synthesis of crystalline nickel oxide nanoparticles. Mater. Chem. Phys. 115, 371–377 (2009). https://doi.org/10.1016/j.matchemphys.2008.12.018
Kievit, F.M., Stephen, Z.R., Veiseh, O., Arami, H., Wang, T.Z., Lai, V.P., Park, J.O., Ellenbogen, R.G., Disis, M.L., Zhang, M.Q.: Targeting of primary breast cancers and metastases in a transgenic mouse model using rationally designed multifunctional SPIONs. ACS Nano 6, 2591–2601 (2012). https://doi.org/10.1021/nn205070h
Laurent, S., Forge, D., Port, M., Roch, A., Robic, C., Elst, L.V., Muller, R.N.: Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 108, 2064–2110 (2008). https://doi.org/10.1021/cr068445e
Li, P., Nan, C.Y., Wei, Z., Lu, J., Peng, Q., Li, Y.: Mn3O4 nanocrystals: facile synthesis, controlled assembly, and application. Chem. Mater. 22, 4232–4236 (2010). https://doi.org/10.1021/cm100831q
Dyachenko, A.G., Ischenko, O.V., Borysenko, M.V., Gaidai, S.V., Yatsymyrskyi, A.V., Tsapyuk, G.G., Pryhunova, O.V., Kostyrko, O.O.: Co–Fe/Al2O3 nanocomposite catalysts of the process of CO2 hydrogenation. Theoret. Exp. Chem. 58, 134–142 (2022). https://doi.org/10.1007/s11237-022-09731-8
Bi, Y., Dalai, A.K.: Selective production of C4 hydrocarbons from syngas using Fe–Co/ZrO2 and SO42−/ZrO2 catalysts. Can. J. Chem. Eng. 81, 230–242 (2003). https://doi.org/10.1002/cjce.5450810208
Kandhasamy, N., Ramalingam, G., Murugadoss, G., Kumar, R.M., Manibalan, G., JothiRamalingam, R., Yadav, H.M.: Copper and zinc oxide anchored silica microsphere: a superior pseudocapacitive positive electrode for aqueous supercapacitor applications. J. Alloys Compd. 888, 161489 (2021). https://doi.org/10.1016/j.jallcom.2021.161489
Sulym, I., Wiśniewska, M., Storozhuk, L., Terpilowski, K., Sternik, D., Borysenko, M., Derylo-Marczewska, A.: Investigation of surface structure, electrokinetic and stability properties of highly dispersed Ho2O3–Yb2O3/SiO2 nanocomposites. Appl. Nanosci. 12, 553–554 (2022). https://doi.org/10.1007/s13204-021-01710-0
Sulym, I., Goncharuk, O., Skwarek, E., Sternik, D., Borysenko, M.V., Derylo-Marczewska, A., Wladyslaw, J., Gun’ko, V.M.: Silica-supported ceria–zirconia and titania–zirconia nanocomposites: structural characteristics and electrosurface properties. Colloids Surf A Physicochem Eng Asp 482, 631–638 (2015). https://doi.org/10.1016/j.colsurfa.2015.07.015
Corrias, A., Casula, M., Ennas, G., Marras, S., Navarra, G., Mountjoy, G.J.: X-ray absorption spectroscopy study of FeCo–SiO2 nanocomposites prepared by the sol–gel method. Phys. Chem. B 107, 3030–3039 (2003). https://doi.org/10.1021/jp027078h
Dyachenko, A., Ischenko, O., Goncharuk, O., Borysenko, M., Mischanchuk, O., Gun’ko, V., Sternik, D., Lisnyak, V.: Preparation and characterization of Ni–Co/SiO2 nanocomposite catalysts for CO2 methanation. Appl. Nanosci. 12, 349–358 (2022). https://doi.org/10.1007/s13204-020-01650-1
Mallampati, R., Valiyaveettil, S.: Biomimetic metal oxides for the extraction of nanoparticles from water. Nanoscale 5, 3395–3399 (2013). https://doi.org/10.1039/C3NR34221B
Gaidai, S.V., Gryn’ko, V.S., Zhludenko, M.G., Dyachenko, A.G., Tkach, V.M., Ishchenko, O.V.: Activity of carbon-fiber-supported Fe–Co catalysts in the CO2 methanation reaction. J. Superhard Mater. 39, 122–128 (2017). https://doi.org/10.3103/S1063457617020071
Gregg, S.J., Sing, K.S.W.: Adsorption, surface area and porosity. Academic Press, London (1982)
Gun’ko, V.M.: Composite materials: textural characteristics. Appl. Surf. Sci. 307, 444–454 (2014). https://doi.org/10.1016/j.apsusc.2014.04.055
JCPDS Database, International Center for Diffraction Data, PA 2001 Available: http://www.icdd.com
Monshi, A., Foroughi, M., Monshi, R.: Modified Sherrer equation to estimate more accurately nano-cristallite size using XRD. World J. Nano Sci. Eng. 2, 154–160 (2012). https://doi.org/10.4236/wjnse.2012.23020
Thommes, M., Kaneko, K., Neimark, A.V., Olivier, J.P., Rodriguez-Reinoso, F., Rouquerol, J., Sing, K.S.W.: Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. (2015). https://doi.org/10.1515/pac-2014-1117
Gun’ko, V.M., Turov, V.V., Zarko, V.I., Goncharuk, O.V., Pakhlov, E.M., Skubiszewska-Zięba, J., Blitz, J.P.: Interfacial phenomena at a surface of individual and complex fumed nanooxides. Adv. Colloid Interface Sci. 235, 108–189 (2016). https://doi.org/10.1016/j.cis.2016.06.003
Senthil, V.P., Gajendiran, J., Raj, S.G., Shanmugavel, T., Ramesh, K.G., Parthasaradhi, R.C.: Study of structural and magnetic properties of cobalt ferrite (CoFe2O4) nanostructures. Chem. Phys. Lett. 695, 19–23 (2018). https://doi.org/10.1016/j.cplett.2018.01.057
Tang, Ch.W., Wang, Ch.B., Chien, Sh.H.: Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS. Thermochim. Acta 473, 68–73 (2008). https://doi.org/10.1016/j.tca.2008.04.015
Kurtan, U., Topkaya, R., Baykal, A., Toprak, M.S.: Temperature dependent magnetic properties of CoFe2O4/CTAB nanocomposite synthesized by sol–gel auto-combustion technique. Ceram. Int. 39, 6551 (2013). https://doi.org/10.1016/j.ceramint.2013.01.088
Waldron, R.D.: Infrared spectra of ferrites. Phys. Rev. 99, 1727–1735 (1955). https://doi.org/10.1103/PhysRev.99.1727
Biesinger, M.C., Payne, B.P., Grosvenor, A.P., Lau, L.W.M., Gerson, A.R., Smart, R.S.C.: Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 257, 2717–2730 (2011). https://doi.org/10.1016/j.apsusc.2010.10.051
Ischenko, O.V., Dyachenko, A.G., Saldan, I., Lisnyak, V.V., Diyuk, V.E., Vakaliuk, A.V., Yatsymyrskyi, A.V., Gaidai, S.V., Zakharova, T.M., Makota, O., Ericsson, T., Häggström, L.: Methanation of CO2 on bulk Co–Fe catalysts. Int. J. Hydrogen Energy 46, 37860–37871 (2021). https://doi.org/10.1016/j.ijhydene.2021.09.034
Mullet, M., Khare, V., Ruby, C.: XPS study of Fe(II)–Fe(III) (oxy)hydroxycarbonate green rust compounds. Surf. Interface Anal. 40, 323–328 (2008). https://doi.org/10.1002/sia.2758
McCafferty, E., Wightman, J.P.: Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 26, 549–564 (1998)
Rui, M., Peng, Yu.: The influences of matrix materials on the magnetic and mechanical properties of Fe78Si9B13 soft magnetic composites fabricated by injection molding. Mater. Res. Bull. 139, 111256 (2021). https://doi.org/10.1016/j.materresbull.2021.111256
Wang, L., Qiao, J., Zheng, W., Cai, Y., Ying, W., Li, S., Che, JYu.: Microstructure and properties of FeSiCr/PA6 composites by injection molding using FeSiCr powders by phosphating and coupling treatment. J. Magn. Magn. Mater. 452, 210–218 (2018). https://doi.org/10.1016/j.jmmm.2017.12.067
Acknowledgements
The authors are grateful for the financial support of the International Visegrad Fund (Dr. Iryna Sulym, Contract number 52210724; D.Sc. Olena Goncharuk, Contract number 52211441) as well as to Professor V.M. Gun’ko for the developing and providing the program for the pore size distribution calculation.
Funding
Alla Dyachenko, Olena Ischenko, Sternik Dariusz, Eugen Pakhlov, Mykola Borysenko, Lyudmila Storozhuk, Lyudmila Andriyko—No funding. Iryna Sulym—International Visegrad Fund, 52210724. Olena Goncharuk—International Visegrad Fund, 52211441. Konrad Terpilowski—International Visegrad Fund, 52211441 and 52210724.
Author information
Authors and Affiliations
Contributions
AD—Conceptualization, Investigation, Writing—original draft. OI—Data curation. IS—SEM, ASAP, Writing—original draft. SD—SEM, ASAP. EP—synthesis, FTIR. MB—XRD. LS—investigation of magnetical properties. LA—particle size distribution. OG—Conceptualization, Investigation, Writing—original draft. KT—Writing—original draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dyachenko, A., Terpilowski, K., Ischenko, O. et al. Co–Fe–oxide nanoparticles supported on the various highly dispersed matrices: the effect of the carrier on structural and magnetic properties. Adsorption 30, 293–311 (2024). https://doi.org/10.1007/s10450-023-00417-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-023-00417-8