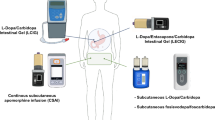
Abstract
The standard of care is a term that refers to the level of care, skill, and treatment that a healthcare provider should offer to a patient based on the current scientific evidence and the level of medical knowledge available in the field. For Parkinson’s disease (PD), the standard care is mostly considered to be oral treatment with dopaminergic drugs, particularly levodopa which remains the ‘gold standard’. However, effective management with levodopa during the later stages of the disease becomes increasingly challenging due to the ongoing neurodegenerative process, the consequences of its pulsatile dopaminergic stimulation, and the gastrointestinal barriers to effective drug absorption. As a result, the concept of applying continuous dopaminergic stimulation has emerged with infusion therapies (continuous subcutaneous apomorphine, levodopa–carbidopa intestinal gel, and levodopa–entacapone–carbidopa intestinal gel infusion). These therapies seek to provide continuous stimulation of striatal dopamine receptors that is efficient not only in alleviating clinical symptoms, but also in delaying, reducing, and possibly preventing the onset of levodopa-related motor (fluctuations, dyskinesia) and non-motor complications; and they are also associated with clinically relevant side effects. Clinical studies and real-life experience support the notion that infusion therapies should be accepted as part of the standard of care for patients with advanced PD who have refractory, severe, and disabling motor complications that affect their quality of life. However, they should be considered based on the needs of individualized patients and the access to these advanced therapies needs to be made more accessible to the general PD population.

Similar content being viewed by others
References
Ahlskog JE, Muenter MD (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Movement Disorders off J Movement Disorder Society 16(3):448–458. https://doi.org/10.1002/mds.1090
Antonini A, Tolosa E (2009) Apomorphine and levodopa infusion therapies for advanced Parkinson’s disease: selection criteria and patient management. Expert Rev Neurother 9(6):859–867. https://doi.org/10.1586/ern.09.48
Antonini A, Poewe W, Chaudhuri KR, Jech R, Pickut B, Pirtošek Z, Szasz J, Valldeoriola F, Winkler C, Bergmann L, Yegin A, Onuk K, Barch D, Odin P, GLORIA study co-investigators, (2017) Levodopa–carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat Disord 45:13–20. https://doi.org/10.1016/j.parkreldis.2017.09.018
Antonini A, Stoessl AJ, Kleinman LS, Skalicky AM, Marshall TS, Sail KR, Onuk K, Odin PLA (2018a) Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr Med Res Opin 34(12):2063–2073. https://doi.org/10.1080/03007995.2018.1502165
Antonini A, Robieson WZ, Bergmann L, Yegin A, Poewe W (2018b) Age/disease duration influence on activities of daily living and quality of life after levodopa–carbidopa intestinal gel in Parkinson’s disease. Neurodegenerative Disease Management 8(3):161–170. https://doi.org/10.2217/nmt-2017-0046
Antonini A, Odin P, Schmidt P, Cubillos F, Standaert DG, Henriksen T, Jimenez-Shahed J, Alobaidi A, Jalundhwala YJ, Bao Y, Zamudio J, Parra JC, Kukreja P, Onuk K, Skalicky AM, Kleinman L, Jones E, Metz S, Fernandez HH (2021) Validation and clinical value of the MANAGE-PD tool: A clinician-reported tool to identify Parkinson’s disease patients inadequately controlled on oral medications. Parkinsonism Relat Disord 92:59–66. https://doi.org/10.1016/j.parkreldis.2021.10.009
Auffret M, Drapier S, Vérin M (2018) Pharmacological insights into the use of Apomorphine in Parkinson’s disease: clinical relevance. Clin Drug Investig 38(4):287–312. https://doi.org/10.1007/s40261-018-0619-3
Cha Y, Park TY, Leblanc P, Kim KS (2023) Current status and future perspectives on stem cell-based therapies for parkinson’s disease. J Movement Disorders 16(1):22–41. https://doi.org/10.14802/jmd.22141
Chaudhuri KR, Leta V (2022) Apomorphine infusion for improving sleep in Parkinson’s disease. The Lancet Neurology 21(5):395–398. https://doi.org/10.1016/S1474-4422(22)00128-4
Chaudhuri KR, Kovács N, Pontieri FE, Aldred J, Bourgeois P, Davis TL, Cubo E, Anca-Herschkovitsch M, Iansek R, Siddiqui MS, Simu M, Bergmann L, Ballina M, Kukreja P, Ladhani O, Jia J, Standaert DG (2023) Levodopa Carbidopa intestinal gel in advanced Parkinson’s disease: DUOGLOBE Final 3-year results. J Parkinsons Dis 13(5):769–783. https://doi.org/10.3233/JPD-225105
Ciurleo R, Corallo F, Bonanno L, Lo Buono V, Di Lorenzo G, Versaci R, Allone C, Palmeri R, Bramanti P, Marino S (2018) Assessment of duodopa® effects on quality of life of patients with advanced Parkinson’s disease and their caregivers. J Neurol 265(9):2005–2014. https://doi.org/10.1007/s00415-018-8951-3
Deuschl G, Antonini A, Costa J, Śmiłowska K, Berg D, Corvol JC, Fabbrini G, Ferreira J, Foltynie T, Mir P, Schrag A, Seppi K, Taba P, Ruzicka E, Selikhova M, Henschke N, Villanueva G, Moro E (2022) European academy of neurology/movement disorder society-European section guideline on the treatment of Parkinson’s disease: I invasive therapies. Move Disorder : off J Movement Disorder Society 37(7):1360–1374. https://doi.org/10.1002/mds.29066
di Biase L, Pecoraro PM, Carbone SP, Caminiti ML, Di Lazzaro V (2023) Levodopa-induced Dyskinesias in Parkinson’s disease: an overview on pathophysiology, clinical manifestations, therapy management strategies and future directions. J Clin Med 12(13):4427. https://doi.org/10.3390/jcm12134427
Fahn, S., Oakes, D., Shoulson, I., Kieburtz, K., Rudolph, A., Lang, A., Olanow, C. W., Tanner, C., Marek, K., Parkinson Study Group (2004). Levodopa and the progression of Parkinson’s disease. The New England journal of medicine, 351 (24), 2498–2508. Doi https://doi.org/10.1056/NEJMoa033447
Giladi N, Gurevich T, Djaldetti R, Adar L, Case R, Leibman-Barak S, Sasson N, Caraco Y (2021) ND0612 (levodopa/carbidopa for subcutaneous infusion) in patients with Parkinson’s disease and motor response fluctuations: a randomized, placebo-controlled phase 2 study. Parkinsonism Relat Disord 91:139–145. https://doi.org/10.1016/j.parkreldis.2021.09.024
Hauser RA, Zeitlin L, Fisher S, D’Souza R (2021) Duration of benefit per dose: Carbidopa-Levodopa immediate release vs. extended release capsules (Rytary®). Parkinsonism Relat Disord 82:133–137. https://doi.org/10.1016/j.parkreldis.2020.12.002
Hely MA, Morris JG, Reid WG, Trafficante R (2005) Sydney multicenter study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Movement Disorders : off J Move Disorder Society 20(2):190–199. https://doi.org/10.1002/mds.20324
Honig H, Antonini A, Martinez-Martin P, Forgacs I, Faye GC, Fox T, Fox K, Mancini F, Canesi M, Odin P, Chaudhuri KR (2009) Intrajejunal levodopa infusion in Parkinson’s disease: a pilot multicentre study of effects on nonmotor symptoms and quality of life. Movem Disorders : off J Move Disorder Society 24(10):1468–1474. https://doi.org/10.1002/mds.22596
Horstink, M., Tolosa, E., Bonuccelli, U., Deuschl, G., Friedman, A., Kanovsky, P., Larsen, J. P., Lees, A., Oertel, W., Poewe, W., Rascol, O., Sampaio, C., European Federation of Neurological Societies, and Movement Disorder Society-European Section (2006). Review of the therapeutic management of Parkinson's disease. Report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES). Part II: late (complicated) Parkinson's disease. European journal of neurology, 13 (11), 1186–1202. Doi https://doi.org/10.1111/j.1468-1331.2006.01548.x
Jenner P (2013) Wearing off, dyskinesia, and the use of continuous drug delivery in Parkinson’s disease. Neurol Clin 31(3 Suppl):S17–S35. https://doi.org/10.1016/j.ncl.2013.04.010
Katzenschlager R, Poewe W, Rascol O, Trenkwalder C, Deuschl G, Chaudhuri KR, Henriksen T, van Laar T, Spivey K, Vel S, Staines H, Lees A (2018) Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. The Lancet Neurology 17(9):749–759. https://doi.org/10.1016/S1474-4422(18)30239-4
Krüger R, Klucken J, Weiss D, Tönges L, Kolber P, Unterecker S, Lorrain M, Baas H, Müller T, Riederer P (2017) Classification of advanced stages of Parkinson’s disease: translation into stratified treatments. J Neural Transmission (vienna, Austria 1996) 124(8):1015–1027. https://doi.org/10.1007/s00702-017-1707-x
Lau, Y. H., Leta, V., Rukavina, K., Parry, M., Ann Natividad, J., Metta, V., Chung-Faye, G., & Chaudhuri, K. R. (2022). Tolerability of overnight rotigotine transdermal patch combined with intrajejunal levodopa infusion at 1 year: a 24-h treatment option in Parkinson’s disease. Journal of neural transmission (Vienna, Austria : 1996), 129 (7), 889–894. Doi https://doi.org/10.1007/s00702-022-02506-4
Leta V, van Wamelen DJ, Sauerbier A, Jones S, Parry M, Rizos A, Chaudhuri KR (2020) Opicapone and Levodopa–carbidopa intestinal gel infusion: the way forward towards cost savings for healthcare systems? J Parkinsons Dis 10(4):1535–1539. https://doi.org/10.3233/JPD-202022
Leta V, Dafsari HS, Sauerbier A, Metta V, Titova N, Timmermann L, Ashkan K, Samuel M, Pekkonen E, Odin P, Antonini A, Martinez-Martin P, Parry M, van Wamelen DJ, Ray Chaudhuri K (2021) Personalised advanced therapies in Parkinson’s disease: the role of non-motor symptoms profile. J Personalized Med 11(8):773. https://doi.org/10.3390/jpm11080773
Leta, V., Klingelhoefer, L., Longardner, K., Campagnolo, M., Levent, H. Ç., Aureli, F., Metta, V., Bhidayasiri, R., Chung-Faye, G., Falup-Pecurariu, C., Stocchi, F., Jenner, P., Warnecke, T., Ray Chaudhuri, K., International Parkinson and Movement Disorders Society Non-Motor Parkinson's Disease Study Group (2023).Gastrointestinal barriers to levodopa transport and absorption in Parkinson’s disease. European journal ofneurology, 30 (5) 1465–1480. Doi https://doi.org/10.1111/ene.15734
Loens S, Chorbadzhieva E, Kleimann A, Dressler D, Schrader C (2017) Effects of levodopa/carbidopa intestinal gel versus oral levodopa/carbidopa on B vitamin levels and neuropathy. Brain and Behavior 7(5):e00698. https://doi.org/10.1002/brb3.698
Lowin J, Sail K, Baj R, Jalundhwala YJ, Marshall TS, Konwea H, Chaudhuri KR (2017) The cost-effectiveness of levodopa/carbidopa intestinal gel compared to standard care in advanced Parkinson’s disease. J Med Econ 20(11):1207–1215. https://doi.org/10.1080/13696998.2017.1379411
Luquin MR, Kulisevsky J, Martinez-Martin P, Mir P, Tolosa ES (2017) Consensus on the definition of advanced Parkinson’s disease: A Neurologists-based delphi study (CEPA Study). Parkinson’s Disease 2017:4047392. https://doi.org/10.1155/2017/4047392
Malek N, Grosset DG (2015) Medication adherence in patients with Parkinson’s disease. CNS Drugs 29(1):47–53. https://doi.org/10.1007/s40263-014-0220-0
Maratos EC, Jackson MJ, Pearce RK, Cannizzaro C, Jenner P (2003) Both short- and long-acting D-1/D-2 dopamine agonists induce less dyskinesia than L-DOPA in the MPTP-lesioned common marmoset (Callithrix jacchus). Exp Neurol 179(1):90–102. https://doi.org/10.1006/exnr.2002.8055
Martinez-Martin, P., Reddy, P., Katzenschlager, R., Antonini, A., Todorova, A., Odin, P., Henriksen, T., Martin, A., Calandrella, D., Rizos, A., Bryndum, N., Glad, A., Dafsari, H. S., Timmermann, L., Ebersbach, G., Kramberger, M. G., Samuel, M., Wenzel, K., Tomantschger, V., Storch, A., Chaudhuri, KR., (2015). EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson's disease. Movement disorders. Official Journal of the Movement Disorder Society, 30 (4) 510–516. Doi https://doi.org/10.1002/mds.26067
Masood N, Jimenez-Shahed J (2023) Effective management of “OFF” episodes in Parkinson’s disease: emerging treatment strategies and unmet clinical needs. Neuropsychiatr Dis Treat 19:247–266. https://doi.org/10.2147/NDT.S273121
Meira B, Degos B, Corsetti E, Doulazmi M, Berthelot E, Virbel-Fleischman C, Dodet P, Méneret A, Mariani LL, Delorme C, Cormier-Dequaire F, Bendetowicz D, Villain N, Tarrano C, Mantisi L, Letrillart H, Louapre C, McGovern E, Worbe Y, Grabli D, Roze E (2021) Long-term effect of apomorphine infusion in advanced Parkinson’s disease: a real-life study. NPJ Parkinson’s Disease 7(1):50. https://doi.org/10.1038/s41531-021-00194-7
Merola A, Romagnolo A, Zibetti M, Bernardini A, Cocito D, Lopiano L (2016) Peripheral neuropathy associated with levodopa–carbidopa intestinal infusion: a long-term prospective assessment. Eur J Neurol 23(3):501–509. https://doi.org/10.1111/ene.12846
Metta V, Batzu L, Leta V, Trivedi D, Powdleska A, Mridula KR, Kukle P, Goyal V, Borgohain R, Chung-Faye G, Chaudhuri KR (2021) Parkinson’s disease: personalized pathway of care for Device-Aided therapies (DAT) and the role of continuous objective monitoring (COM) using wearable sensors. J Personalized Med 11(7):680. https://doi.org/10.3390/jpm11070680
Nakmode DD, Day CM, Song Y, Garg S (2023) The management of Parkinson’s disease: an overview of the current advancements in drug delivery systems. Pharmaceutics 15(5):1503. https://doi.org/10.3390/pharmaceutics15051503
Nyholm D. (2007). The rationale for continuous dopaminergic stimulation in advanced Parkinson's disease.Parkinsonism and related disorders, 13 Suppl, S13–S17. Doi https://doi.org/10.1016/j.parkreldis.2007.06.005
Odin P, Ray Chaudhuri K, Slevin JT, Volkmann J, Dietrichs E, Martinez-Martin P, Krauss JK, Henriksen T, Katzenschlager R, Antonini A, Rascol O, Poewe W, Committees NS (2015) Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: consensus from an international survey and discussion program. Parkinsonism Relat Disord 21(10):1133–1144. https://doi.org/10.1016/j.parkreldis.2015.07.020
Olanow CW, Obeso JA, Stocchi F (2006) Drug insight: Continuous dopaminergic stimulation in the treatment of Parkinson’s disease. Nat Clin Pract Neurol 2(7):382–392. https://doi.org/10.1038/ncpneuro0222
Olanow, C. W., Kieburtz, K., Odin, P., Espay, A. J., Standaert, D. G., Fernandez, H. H., Vanagunas, A., Othman, A. A., Widnell, K. L., Robieson, W. Z., Pritchett, Y., Chatamra, K., Benesh, J., Lenz, R. A., Antonini, A., LCIG Horizon Study Group (2014). Continuous intrajejunal infusion of levodopa–carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study. The Lancet. Neurology, 13 (2), 141–149. Doi https://doi.org/10.1016/S1474-4422(13)70293-X
Olanow, CW., Kieburtz, K., Rascol, O., Poewe, W., Schapira, A. H., Emre, M., Nissinen, H., Leinonen, M., Stocchi, F., Stalevo Reduction in Dyskinesia Evaluation in Parkinson’s Disease (STRIDE-PD) Investigators (2013). Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Movement disorders : Official Journal of the Movement Disorder Society, 28 (8), 1064–1071. https://doi.org/10.1002/mds.25364
Pahwa, R., Factor, S. A., Lyons, K. E., Ondo, W. G., Gronseth, G., Bronte-Stewart, H., Hallett, M., Miyasaki, J.,Stevens, J., Weiner, W. J., Quality Standards Subcommittee of the American Academy of Neurology (2006). Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology, 66 (7), 983–995. Doi https://doi.org/10.1212/01.wnl.0000215250.82576.87
Pirtošek Z, Bajenaru O, Kovács N, Milanov I, Relja M, Skorvanek M (2020) Update on the management of Parkinson’s disease for General Neurologists. Parkinson’s Disease 2020:9131474. https://doi.org/10.1155/2020/9131474
Rascol, O., Tönges, L., deVries, T., Jaros, M., Quartel, A., Jacobs, D., Allay-LID I and ALLAY-LID II study groups (2022). Immediate-release/extended-release amantadine (OS320) to treat Parkinson's disease with levodopa-induced dyskinesia: Analysis of the randomized, controlled ALLAY-LID studies. Parkinsonism and related disorders, 96, 65–73. Doi https://doi.org/10.1016/j.parkreldis.2022.01.022
Regensburger, M., Ip, C. W., Kohl, Z., Schrader, C., Urban, P. P., Kassubek, J., Jost, W. H. (2023). Clinical benefit of MAO-B and COMT inhibition in Parkinson’s disease: practical considerations. Journal of neural transmission (Vienna, Austria : 1996), 130 (6), 847–861. Doi https://doi.org/10.1007/s00702-023-02623-8
Rosebraugh M, Voight EA, Moussa EM, Jameel F, Lou X, Zhang GGZ, Mayer PT, Stolarik D, Carr RA, Enright BP, Liu W, Facheris MF, Kym PR (2021) Foslevodopa/Foscarbidopa: a new subcutaneous treatment for Parkinson’s disease. Ann Neurol 90(1):52–61. https://doi.org/10.1002/ana.26073
Schuepbach, W. M., Rau, J., Knudsen, K., Volkmann, J., Krack, P., Timmermann, L., Hälbig, T. D., Hesekamp, H., Navarro, S. M., Meier, N., Falk, D., Mehdorn, M., Paschen, S., Maarouf, M., Barbe, M. T., Fink, G. R., Kupsch, A., Gruber, D., Schneider, G. H., Seigneuret, E., EARLYSTIM Study Group (2013). Neurostimulation for Parkinson's disease with early motor complications. The New England journal of medicine, 368 (7), 610–622. Doi https://doi.org/10.1056/NEJMoa1205158
Senek M, Nielsen EI, Nyholm D (2017) Levodopa-entacapone-carbidopa intestinal gel in Parkinson’s disease: a randomized crossover study. Movement Disord off J Move Disorder Society 32(2):283–286. https://doi.org/10.1002/mds.26855
Shoulson I, Glaubiger GA, Chase TN (1975) On-off response Clinical and biochemical correlations during oral and intravenous levodopa administration in parkinsonian patients. Neurology 25(12):1144–1148. https://doi.org/10.1212/wnl.25.12.1144
Stibe C, Lees A, Stern G (1987) Subcutaneous infusion of apomorphine and lisuride in the treatment of parkinsonian on-off fluctuations. Lancet (london, England) 1(8537):871. https://doi.org/10.1016/s0140-6736(87)91660-6
Stocchi F (2006) The levodopa wearing-off phenomenon in Parkinson’s disease: pharmacokinetic considerations. Expert Opin Pharmacother 7(10):1399–1407. https://doi.org/10.1517/14656566.7.10.1399
Stocchi F, Olanow CW (2004) Continuous dopaminergic stimulation in early and advanced Parkinson’s disease. Neurology 62(1 Suppl 1):S56–S63. https://doi.org/10.1212/wnl.62.1_suppl_1.s56
Stocchi F, Rascol O, Kieburtz K, Poewe W, Jankovic J, Tolosa E, Barone P, Lang AE, Olanow CW (2010) Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: the STRIDE-PD study. Ann Neurol 68(1):18–27. https://doi.org/10.1002/ana.22060
Straka I, Minár M, Gažová A, Valkovič P, Kyselovič J (2018) Clinical aspects of adherence to pharmacotherapy in Parkinson disease: A PRISMA-compliant systematic review. Medicine 97(23):e10962. https://doi.org/10.1097/MD.0000000000010962
Tall, P., Qamar, M. A., Batzu, L., Leta, V., Falup-Pecurariu, C., & Ray Chaudhuri, K. (2023). Non-oral continuous drug delivery based therapies and sleep dysfunction in Parkinson's disease. Journal of neural transmission (Vienna, Austria : 1996), https://doi.org/10.1007/s00702-023-02640-7. Advance online publication. Doi https://doi.org/10.1007/s00702-023-02640-7
Timpka J, Nitu B, Datieva V, Odin P, Antonini A (2017) Device-aided treatment strategies in advanced Parkinson’s Disease. Int Rev Neurobiol 132:453–474. https://doi.org/10.1016/bs.irn.2017.03.001
Titova N, Ray Chaudhuri K (2017) Intrajejunal levodopa infusion therapy for Parkinson’s disease:practical and pragmatic tips for successful maintenance of therapy. Expert Rev Neurother 17(6):529–537. https://doi.org/10.1080/14737175.2017.1317595
Van Laar AD, Van Laar VS, San Sebastian W, Merola A, Elder JB, Lonser RR, Bankiewicz KS (2021) An update on gene therapy approaches for Parkinson’s disease: restoration of dopaminergic function. J Parkinsons Dis 11(s2):S173–S182. https://doi.org/10.3233/JPD-212724
Wright BA, Waters CH (2013) Continuous dopaminergic delivery to minimize motor complications in Parkinson’s disease. Expert Rev Neurother 13(6):719–729. https://doi.org/10.1586/ern.13.47
Zagnoli, F., Leblanc, A., Viakhireva-Dovganyuk, I., Delabrousse-Mayoux, J. P., Pouyet, A., Ziegler, M., Sogni,L., Patat, M., Bouillot, R., Vérin, M., APOKADO Group (2023). Feasibility and benefits of home initiation of subcutaneous apomorphine infusion for patients with Parkinson's disease: the APOKADO study. Journal of neural transmission (Vienna, Austria : 1996), 1–12. Advance online publication. Doi https://doi.org/10.1007/s00702-023-02609-6
Acknowledgements
The views expressed are those of the authors and not necessarily those of the NHS, NIHR, or Departments of Health. The figure of this manuscript was created with Biorender.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ZP reports honoraria from Britannia, Abbvie, and Stada. VL reports grants from Parkinson’s UK and honoraria from UCB, Bial, Invisio, Profile, AbbVie, and Britannia Pharmaceuticals, outside the submitted work. PJ received honoraria for consultancies, advisory boards, and sponsored lectures from Abbvie, Britannia Pharmaceuticals, Eisai, FP Pharmaceuticals, Kyowa Kirin, Hofman La Roche, Profile Pharma, Tabuk Pharmaceuticals, UCB, Worldwide Clinical Trials and Zambon, outside of the submitted work. MV reports grants from France Parkinson, INCR, BAA and CECAP and honoraria from Britannia, Abbvie, Medtonic, Adelia Medical, Asdia, Medtronic, Boston Scientific, Sanofi, Aguettant.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pirtošek, Z., Leta, V., Jenner, P. et al. Should continuous dopaminergic stimulation be a standard of care in advanced Parkinson’s disease?. J Neural Transm 130, 1395–1404 (2023). https://doi.org/10.1007/s00702-023-02708-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-023-02708-4