Abstract
Purpose of Review
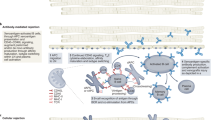
The first successful pig to human cardiac xenotransplantation in January 2022 represented a major step forward in the fields of heart failure, immunology, and applied genetic engineering, using a 10-gene edited (GE) pig. This review summarizes the evolution of preclinical modelling data which informed the use of each of the 10 genes modified in the 10-GE pig: GGTA1, Β4GalNT2, CMAH, CD46, CD55, TBM, EPCR, CD47, HO-1, and growth hormone receptor.
Recent Findings
The translation of the 10-GE pig from preclinical modelling to clinical compassionate xenotransplant use was the culmination of decades of research combating rejection, coagulopathy, inflammation, and excessive xenograft growth.
Summary
Understanding these 10 genes with a view to their combinatorial effects will be useful in anticipated xenotransplant clinical trials.
Similar content being viewed by others
Abbreviations
- EPCR:
-
Endothelial protein C receptor
- Gal:
-
Galactose-alpha-1,3-galactose
- GE:
-
Genetically engineered
- GHR:
-
Growth hormone receptor
- GHRKO:
-
Growth hormone receptor knockout
- HAR:
-
Hyperacute rejection
- HO-1:
-
Heme oxygenase-1
- KO:
-
Knockout
- Neu5Gc:
-
N-glycolylneuraminic acid
- NHP:
-
Non-human primate
- SDa:
-
SD antigen
- TBM:
-
Thrombomodulin
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118(17):3272–87.
Kilic A, Mathier MA, Hickey GW, Sultan I, Morell VO, Mulukutla SR, et al. Evolving trends in adult heart transplant with the 2018 heart allocation policy change. JAMA Cardiol. 2021;6(2):159–167.
Griffith BP, Goerlich CE, Singh AK, Rothblatt M, Lau CL, Shah A, et al. Genetically modified porcine-to-human cardiac xenotransplantation. N Engl J Med. 2022;387(1):35–44.
•• Mohiuddin MM, Singh AK, Scobie L, Goerlich CE, Grazioli A, Saharia K, et al. Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: a case report. Lancet. 2023. This is the definitive postmortem analysis from the first successful pig to human solid organ xenotransplant, reviewing both the evidence for success as well as areas for further investigation.
Cooper DKC, Hara H, Iwase H, Yamamoto T, Li Q, Ezzelarab M, et al. Justification of specific genetic modifications in pigs for clinical organ xenotransplantation. Xenotransplantation. 2019;26(4):e12516.
• Singh AK, Goerlich CE, Shah AM, Zhang T, Tatarov I, Ayares D, et al. Cardiac xenotransplantation: progress in preclinical models and prospects for clinical translation. Transpl Int. 2022;35:10171. This paper reviews the crucial breakthroughs that have sprung from pre-clinical modelling and previews future directions of xenotransplantation study.
Niu D, Wei HJ, Lin L, George H, Wang T, Lee IH, et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 2017;357(6357):1303–7.
Ladowski JM, Martens GR, Reyes LM, Hauptfeld-Dolejsek V, Tector M, Tector J. Examining epitope mutagenesis as a strategy to reduce and eliminate human antibody binding to class II swine leukocyte antigens. Immunogenetics. 2019;71(7):479–487.
Schuurman H, Cheng J, Lam T. Pathology of xenograft rejection: a commentary. Xenotransplantation (Københaven). 2003;10(4):293–9.
Cooper DKC, Ekser B, Tector AJ. Immunobiological barriers to xenotransplantation. Int J Surg. 2015;23:211–6.
Singh AK, Chan JL, DiChiacchio L, Hardy NL, Corcoran PC, Lewis BGT, et al. Cardiac xenografts show reduced survival in the absence of transgenic human thrombomodulin expression in donor pigs. Xenotransplantation. 2019;26(2):e12465.
Simon PM, Neethling FA, Taniguchi S, Goode PL, Zopf D, Hancock WW, et al. Intravenous infusion of Galα1–3Gal oligosaccharides in baboons delays hyperacute rejection of porcine heart xenografts. Transplantation. 1998;65(3):346–353.
Azimzadeh A, Meyer C, Watier H, Beller J, Chenard-Neu M, Kieny R, et al. Removal of primate xenoreactive natural antibodies by extracorporeal perfusion of pig kidneys and livers. Transpl Immunol. 1998;6(1):13–22.
Cooper DKC, Kuwaki K, Tseng Y, Dor, Frank J M F, Shimizu A, Houser SL, et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11(1):29–31.
Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen S, et al. Production of α1,3-galactosyltransferase: deficient pigs. Science (American Association for the Advancement of Science). 2003;299(5605):411–414.
Galili U. Induced anti-non gal antibodies in human xenograft recipients. Transplantation. 2012;93(1):11–16.
McGregor CG, Ricci D, Miyagi N, Stalboerger PG, Du Z, Oehler EA, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012;93(7):686–92.
Zhao C, Cooper DKC, Dai Y, Hara H, Cai Z, Mou L. The Sda and Cad glycan antigens and their glycosyltransferase, β1,4GalNAcT‐II, in xenotransplantation. Xenotransplantation (Københaven). 2018;25(2):e12386-n/a.
Byrne GW, Stalboerger PG, Du Z, Davis TR, McGregor CG. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation. 2011;91(3):287–92.
Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA 1/ CMAH /β4Gal NT 2 genes. Xenotransplantation (Københaven). 2015;22(3):194–202.
Altman MO, Gagneux P. Absence of Neu5Gc and presence of anti-Neu5Gc antibodies in humans—an evolutionary perspective. Front Immunol. 2019;10:789.
Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation (Københaven). 2002;9(6):376–81.
Burlak C, Paris LL, Lutz AJ, Sidner RA, Estrada J, Li P, et al. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am J Transplant. 2014;14(8):1895–900.
Wang Z, Li P, Butler JR, Blankenship RL, Downey SM, Montgomery JB, et al. Immunogenicity of renal microvascular endothelial cells from genetically modified pigs. Transplantation. 2016;100(3):533–537.
Yamamoto T, Iwase H, Patel D, Jagdale A, Ayares D, Anderson D, et al. Old World Monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (Triple-Knockout). Sci Rep. 2020;10(1):9771.
Diamond LE, Quinn CM, Martin MJ, Lawson J, Platt JL, Logan JS. A human CD46 transgenic pig model system for the study of discordant xenotransplantation. Transplantation. 2001;71(1):132–42.
Brodbeck WG, Kuttner-Kondo L, Mold C, Medof ME. Structure/function studies of human decay-accelerating factor. Immunology. 2000;101(1):104–11.
Shimizu I, Smith NR, Guiling Z, Medof E, Sykes M. Decay-accelerating factor prevents acute humoral rejection induced by low levels of anti-αGal natural antibodies. Transplantation. 2006;81(1):95–100.
Schuurman H, Pino-Chavez G, Phillips MJ, Thomas L, White DJG, Cozzi E. Incidence of hyperacute rejection in pig-to-primate transplantation using organs from hDAF-transgenic donors. Transplantation. 2002;73(7):1146–1151.
Waterworth PD, Cozzi E, Tolan MJ, Langford G, Braidley P, Chavez G, et al. Pig-to-primate cardiac xenotransplantation and cyclophosphamide therapy. Transplant Proc. 1997;29(1):899–900.
Azimzadeh AM, Kelishadi SS, Ezzelarab MB, Singh AK, Stoddard T, Iwase H, et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein. Xenotransplantation (Københaven). 2015;22(4):310–6.
Miyagawa S, Shirakura R, Iwata K, Nakata S, Matsumiya G, Izutani H, et al. Effects of transfected complement regulatory proteins, MCP, DAF, and MCP/DAE hybrid, on complement-mediated swine endothelial cell lysis. Transplantation. 1994;58(7):834–40.
Fischer K, Kraner-Scheiber S, Petersen B, Rieblinger B, Buermann A, Flisikowska T, et al. Efficient production of multi-modified pigs for xenotransplantation by ‘combineering’, gene stacking and gene editing. Sci Rep. 2016;6(1):29081.
Byrne GW, Mccurry KR, Martin MJ, Mcclellan SM, Platt JL, Logan JS. Transgenic pigs expressing human CD59 and decay-accelerating factor produce an intrinsic barrier to complement-mediated damage. Transplantation. 1997;63(1):149–155.
Johnson S, Brooks NJ, Smith RA, Lea SM, Bubeck D. Structural basis for recognition of the pore-forming toxin intermedilysin by human complement receptor CD59. Cell Rep. 2013;3(5):1369–77.
Aigner B, Klymiuk N, Wolf E. Transgenic pigs for xenotransplantation: selection of promoter sequences for reliable transgene expression. Curr Opin Organ Transplant. 2010;15(2):201–6.
Weiler H, Isermann BH. Thrombomodulin. J Thromb Haemost. 2003;1(7):1515–24.
Salvaris EJ, Moran CJ, Roussel JC, Fisicaro N, Robson SC, Cowan PJ. Pig endothelial protein C receptor is functionally compatible with the human protein C pathway. Xenotransplantation. 2020;27(2):e12557.
Crikis S, Zhang XM, Dezfouli S, Dwyer KM, Murray-Segal LM, Salvaris E, et al. Antiinflammatory and anticoagulant effects of transgenic expression of human thrombomodulin in mice. Am J Transplant. 2010;10(2):242–50.
Mohiuddin MM, Singh AK, Corcoran PC, Thomas Iii ML, Clark T, Lewis BG, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138.
Iwase H, Hara H, Ezzelarab M, Li T, Zhang Z, Gao B, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24(2).
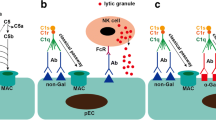
Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104(12):5062–6.
Yan JJ, Koo TY, Lee HS, Lee WB, Kang B, Lee JG, et al. Role of human CD200 overexpression in pig-to-human xenogeneic immune response compared with human CD47 overexpression. Transplantation. 2018;102(3):406–16.
Tena A, Sachs DH, Mallard C, Yang Y, Tasaki M, Farkash E, et al. Prolonged survival of pig skin on baboons following administration of pig cells expressing human CD47. Transplantation. 2017;101(2):316–321.
Takeuchi K, Ariyoshi Y, Shimizu A, Okumura Y, Cara-Fuentes G, Garcia GE, et al. Expression of human CD47 in pig glomeruli prevents proteinuria and prolongs graft survival following pig-to-baboon xenotransplantation. Xenotransplantation. 2021;28(6):e12708.
Camara NO, Soares MP. Heme oxygenase-1 (HO-1), a protective gene that prevents chronic graft dysfunction. Free Radic Biol Med. 2005;38(4):426–35.
Petersen B, Ramackers W, Lucas-Hahn A, Lemme E, Hassel P, Queißer A, et al. Transgenic expression of human heme oxygenase-1 in pigs confers resistance against xenograft rejection during ex vivo perfusion of porcine kidneys. Xenotransplantation (Københaven). 2011;18(6):355–68.
Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4(9):1073–7.
Goerlich CE, Griffith B, Hanna P, Hong SN, Ayares D, Singh AK, et al. The growth of xenotransplanted hearts can be reduced with growth hormone receptor knockout pig donors. J Thorac Cardiovasc Surg. 2023;165(2):e69–81.
Hinrichs A, Riedel EO, Klymiuk N, Blutke A, Kemter E, Längin M, et al. Growth hormone receptor knockout to reduce the size of donor pigs for preclinical xenotransplantation studies. Xenotransplantation. 2021;28(2):e12664.
Troncoso R, Ibarra C, Vicencio JM, Jaimovich E, Lavandero S. New insights into IGF-1 signaling in the heart. Trends Endocrinol Metab. 2014;25(3):128–137.
Mohiuddin MM, Singh AK, Scobie L, Goerlich CE, Grazioli A, Saharia K, et al. Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: a case report. Lancet. 2023;402(10399):397–410.
Montgomery RA, Stern JM, Lonze BE, Tatapudi VS, Mangiola M, Wu M, et al. Results of two cases of pig-to-human kidney xenotransplantation. N Engl J Med. 2022;386(20):1889–98.
Porrett PM, Orandi BJ, Kumar V, Houp J, Anderson D, Cozette Killian A, et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am J Transplant. 2022;22(4):1037–53.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Ayares is the President and CEO of Revivicor and reports other from Revivicor Inc. and personal fees from Revivicor Inc. In addition, Dr. Ayares has a patent on Multitransgenic pigs for xenotransplantation issued. Dr. Mohiuddin reports research funding and pigs provided by United Therapeutics, Inc. and drugs provided by Eledon Pharmaceuticals, Tonix Pharmaceuticals, and Kiniksa Pharmaceuticals. In addition, Dr. Mohiuddin has a patent pending with Kiniksa Pharmaceuticals on the Use of drug KPL 404 to overcome rejection. The other authors have no disclosures to report.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
IRB Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singireddy, S., Tully, A., Galindo, J. et al. Genetic Engineering of Donor Pig for the First Human Cardiac Xenotransplantation: Combatting Rejection, Coagulopathy, Inflammation, and Excessive Growth. Curr Cardiol Rep 25, 1649–1656 (2023). https://doi.org/10.1007/s11886-023-01978-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-023-01978-4