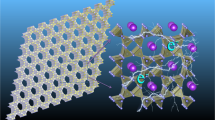
Abstract
The utilization of two-dimensional porous materials as anodes in ion batteries has garnered significant interest within the field of clean energy because of their flexible architecture, high conductivity, rapid diffusion process and high specific ion capacity. Herein, we developed a new metal-free 2D porous compound, namely, B4O2. The stability of the B4O2 monolayer was verified through the ab-initio molecular dynamics simulations and phonon spectrum calculations. The results demonstrate that the adsorption of K, Na, and Li atoms onto the B4O2 monolayer surface is remarkably stable, with all three species exhibiting a shared diffusion path. Specifically, we found that the adsorption of K atoms on the B4O2 monolayer surpasses that of Na and Li atoms, and the diffusion of K atoms occurs at a faster rate than Na and Li atoms on the same monolayer surface. The maximum theoretical specific capacity of K+, Na+ and Li+ is calculated to be 626.1 mAh/g. In addition, the B4O2 monolayer retains good electronic conductivity and electron activity during the atomic adsorption processes. Based on our findings, the B4O2 monolayer exhibits significant potential as anode material for ion batteries. This study paves the way for a novel approach in designing new 2D porous materials specifically tailored for energy storage and conversion applications.
Graphical Abstract







Similar content being viewed by others
References
Hong, X., Wang, R., Liu, Y., Fu, J., Liang, J., Dou, S.: Recent advances in chemical adsorption and catalytic conversion materials for Li–S batteries. J. Energy Chem. 42, 144–168 (2020). https://doi.org/10.1016/j.jechem.2019.07.001
Román-Ramírez, L., Marco, J.: Design of experiments applied to lithium-ion batteries: A literature review. Appl. Energy 320, 119305 (2022). https://doi.org/10.1016/j.apenergy.2022.119305
Kanimozhi, G., Naresh, N., Kumar, H., Satyanarayana, N.: Review on the recent progress in the nanocomposite polymer electrolytes on the performance of lithium-ion batteries. Int. J. Energy Res. 46(6), 7137–7174 (2022). https://doi.org/10.1002/er.7740
Evarts, E.C.: Lithium batteries: To the limits of lithium. Nature 526(7575), S93–S95 (2015). https://doi.org/10.1038/526s93a
Lim, Y.E., Choi, W.S., Kim, J.H., Ahn, Y.N., Kim, I.T.: The Sn–red P-Fe–based alloy materials for efficient Li–ion battery anodes. J. Ind. Eng. Chem. (2023). https://doi.org/10.1016/j.jiec.2023.01.033
Khan, M.I., Nadeem, G., Majid, A., Shakil, M.: A DFT study of bismuthene as anode material for alkali-metal (Li/Na/K)-ion batteries. Mater. Sci. Eng., B 266, 115061 (2021). https://doi.org/10.1016/j.mseb.2021.115061
Zhang, L., Wang, W., Lu, S., Xiang, Y.: Carbon anode materials: a detailed comparison between Na-ion and K-ion batteries. Adv. Energy Mater. 11(11), 2003640 (2021)
Butt, M.K., Rehman, J., Yang, Z., Wang, S., El-Zatahry, A., Alofi, A.S.: Storage of Na in 2D SnS for Na ion batteries a DFT prediction. Phys Chem Chem Phys 24(48), 29609–29615 (2022). https://doi.org/10.1039/d2cp02780a
Chen, H., Lv, P., Liu, Q., Tian, P., Cao, S., Yuan, S.: Bonding iron chalcogenides in a hierarchical structure for high-stability sodium storage. J. Colloid Interface Sci. (2023). https://doi.org/10.1016/j.jcis.2023.01.056
Wu, S., Feng, Y., Jiang, W., Wu, K., Guo, Z., Xiong, D., He, M.: Reduced graphene oxide coated modified SnO2 forms excellent potassium storage properties. Ceramics Int (2023). https://doi.org/10.1016/j.ceramint.2023.01.168
Schaibley, J.R., Yu, H., Clark, G., Rivera, P., Ross, J.S., Seyler, K.L., Xu, X.: Valleytronics in 2D materials. Nat Rev Mater 1(11), 1–15 (2016). https://doi.org/10.1038/natrevmats.2016.55
Deng, D., Novoselov, K., Fu, Q., Zheng, N., Tian, Z., Bao, X.: Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 11(3), 218–230 (2016). https://doi.org/10.1038/nnano.2015.340
Mao, J., Zhou, T., Zheng, Y., Gao, H.: Two-dimensional nanostructures for sodium-ion battery anodes. J Mater Chem A 6(8), 3284–3303 (2018). https://doi.org/10.1039/c7ta10500b
Xia, F., Wang, H., Xiao, D., Dubey, M., Ramasubramaniam, A.: Two-dimensional material nanophotonics. Nat. Photonics 8(12), 899–907 (2014). https://doi.org/10.1038/nphoton.2014.271
Zhong, C., Wu, W., He, J., Ding, G., Liu, Y., Li, D., Zhang, G.: Two-dimensional honeycomb borophene oxide: strong anisotropy and nodal loop transformation. Nanoscale 11(5), 2468–2475 (2019). https://doi.org/10.1039/C8NR08729F
Zhong, C., Feng, C.: An ideal two-dimensional nodal-ring semimetal in tetragonal borophene oxide. Phys. Chem. Chem. Phys. 23(32), 17348–17353 (2021). https://doi.org/10.1039/D1CP02003J
Lin, S., Guo, Y., Xu, M., Zhao, J., Liang, Y., Yuan, X., Li, Y.: AB 2 N monolayer: a direct band gap semiconductor with high and highly anisotropic carrier mobility. Nanoscale 14(3), 930–938 (2022)
Gao, S., Wei, F., Jia, B., Chen, C., Wu, G., Hao, J., Lu, P.: Two-dimensional van der Waals layered VSi2N4 as anode materials for alkali metal (Li, Na and K) ion batteries. J Phys Chem Solid 178, 111339 (2023)
Kasprzak, G.T., Szczesniak, R., Durajski, A.P.: Computational insight into bilayer NC7 anode material for Li/Na/Mg-ion batteries. Comput. Mater. Sci. 225, 112194 (2023)
Wang, S., Wu, Y., Ye, X., Sun, S.: Predict low energy structures of BSi monolayer as high-performance Li/Na/K ion battery anode. Appl. Surf. Sci. 609, 155222 (2023). https://doi.org/10.1016/j.apsusc.2022.155222
Wang, Y., Li, Y.: Ab initio prediction of two-dimensional Si3C enabling high specific capacity as an anode material for Li/Na/K-ion batteries. J Mater Chem A 8(8), 4274–4282 (2020). https://doi.org/10.1039/c9ta11589g
Zhou, Y., Zhao, M., Chen, Z.W., Shi, X.M., Jiang, Q.: Potential application of 2D monolayer β-GeSe as an anode material in Na/K ion batteries. Phys. Chem. Chem. Phys. 20(48), 30290–30296 (2018). https://doi.org/10.1039/c8cp05484c
Yang, M., Kong, F., Chen, L., Tian, B., Guo, J.: Potential application of two-dimensional PC6 monolayer as an anode material in alkali metal-ion (Li, Na, K) batteries. Thin Solid Films (2023). https://doi.org/10.1016/j.tsf.2023.139734
Xia, X., Yin, H., Zhang, Y., Huang, S.: Boron-doped g-CN monolayer as a promising anode for Na/K-ion batteries. Surf Interf 36, 102479 (2023). https://doi.org/10.1016/j.surfin.2022.102479
Wang, Z.-Q., Lü, T.-Y., Wang, H.-Q., Feng, Y.P., Zheng, J.-C.: Review of borophene and its potential applications. Front. Phys. 14, 1–20 (2019)
Xie, S.-Y., Wang, Y., Li, X.-B.: Flat boron: a new cousin of graphene. Adv. Mater. 31(36), 1900392 (2019)
Li, D., Gao, J., Cheng, P., He, J., Yin, Y., Hu, Y., Zhao, J.: 2D boron sheets: structure, growth, and electronic and thermal transport properties. Adv Funct Mater 30(8), 1904349 (2020)
Wang, Q., Fan, G., Xu, H., Tu, X., Wang, X., Chu, X.: C-doped boron nitride nanotubes for the catalysis of acetylene hydrochlorination: a density functional theory study. Mol Cataly 488, 110853 (2020)
Wu, X., Dai, J., Zhao, Y., Zhuo, Z., Yang, J., Zeng, X.C.: Two-dimensional boron monolayer sheets. ACS nano 6(8), 7443–7453 (2012)
Feng, B., Zhang, J., Liu, R.-Y., Iimori, T., Lian, C., Li, H.: Direct evidence of metallic bands in a monolayer boron sheet. Phys Rev B 94(4), 041408 (2016)
Mu, Y., Chen, Q., Chen, N., Lu, H., Li, S.-D.: A novel borophene featuring heptagonal holes: a common precursor of borospherenes. Phys. Chem. Chem. Phys. 19(30), 19890–19895 (2017)
Zhou, X.-F., Oganov, A.R., Wang, Z., Popov, I.A., Boldyrev, A.I., Wang, H.-T.: Two-dimensional magnetic boron. Phys Rev B 93(8), 085406 (2016)
Tkachenko, N.V., Steglenko, D., Fedik, N., Boldyreva, N.M., Minyaev, R.M., Minkin, V.I., Boldyrev, A.I.: Superoctahedral two-dimensional metallic boron with peculiar magnetic properties. Phys. Chem. Chem. Phys. 21(36), 19764–19771 (2019)
Penev, E.S., Kutana, A., Yakobson, B.I.: Can two-dimensional boron superconduct? Nano Lett. 16(4), 2522–2526 (2016)
Gao, M., Li, Q.-Z., Yan, X.-W., Wang, J.: Prediction of phonon-mediated superconductivity in borophene. Phys. Rev. B 95(2), 024505 (2017)
Zhao, Y., Zeng, S., Ni, J.: Superconductivity in two-dimensional boron allotropes. Phys. Rev. B 93(1), 014502 (2016)
Feng, B., Zhang, J., Ito, S., Arita, M., Cheng, C., Chen, L.: Discovery of 2D anisotropic Dirac cones. Adv. Mater. 30(2), 1704025 (2018)
Zhang, H., Xie, Y., Zhang, Z., Zhong, C., Li, Y., Chen, Z., Chen, Y.: Dirac nodal lines and tilted semi-Dirac cones coexisting in a striped boron sheet. J Phys Chem Lett 8(8), 1707–1713 (2017)
Mannix, A.J., Kiraly, B., Hersam, M.C., Guisinger, N.P.: Synthesis and chemistry of elemental 2D materials. Nat. Rev. Chem. 1(2), 0014 (2017)
Gao, B., Gao, P., Lu, S., Lv, J., Wang, Y., Ma, Y.: Interface structure prediction via CALYPSO method. Sci Bull 64(5), 301–309 (2019). https://doi.org/10.1016/j.scib.2019.02.009
Wang, Y., Lv, J., Zhu, L., Ma, Y.: CALYPSO: A method for crystal structure prediction. Comput. Phys. Commun. 183(10), 2063–2070 (2012). https://doi.org/10.1016/j.cpc.2012.05.008
Wang, Y., Lv, J., Zhu, L., Ma, Y.: Crystal structure prediction via particle-swarm optimization. Phys. Rev. B 82(9), 094116 (2010). https://doi.org/10.1103/PhysRevB.82.094116
Kohn, W., Sham, L.J.: Self-consistent equations including exchange and correlation effects. Phys. Rev. 140(4A), A1133 (1965). https://doi.org/10.1103/physrev.140.a1133
Hohenberg, P., Kohn, W.: Inhomogeneous electron gas. Phys Rev 136(3B), B864 (1964). https://doi.org/10.1103/PhysRev.136.B864
Perdew, J.P., Burke, K., Ernzerhof, M.: Generalized gradient approximation made simple. Phys. Rev. Lett. 77(18), 3865 (1996). https://doi.org/10.1103/physrevlett.77.3865
Grimme, S.: Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27(15), 1787–1799 (2006). https://doi.org/10.1002/jcc.20495
Henkelman, G., Uberuaga, B.P., Jónsson, H.: A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113(22), 9901–9904 (2000). https://doi.org/10.1063/1.1329672
Sheppard, D., Terrell, R., Henkelman, G.: Optimization methods for finding minimum energy paths. J. Chem. Phys. 128(13), 134106 (2008). https://doi.org/10.1063/1.2841941
Henkelman, G., Jónsson, H.: Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113(22), 9978–9985 (2000). https://doi.org/10.1063/1.1323224
Nosé, S.: A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81(1), 511–519 (1984). https://doi.org/10.1063/1.447334
Hoover, W.G.: Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 31(3), 1695 (1985). https://doi.org/10.1103/physreva.31.1695
Wang, V., Xu, N., Liu, J.-C., Tang, G., Geng, W.-T.: VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033 (2021). https://doi.org/10.1016/j.cpc.2021.108033
Peng, C., Mercer, M.P., Skylaris, C.-K., Kramer, D.: Lithium intercalation edge effects and doping implications for graphite anodes. J Mater Chem A 8(16), 7947–7955 (2020)
Ma, Y., Xu, S., Fan, X., Singh, D.J., Zheng, W.: Adsorption of K Ions on Single-Layer GeC for Potential Anode of K Ion Batteries. Nanomaterials 11(8), 1900 (2021). https://doi.org/10.3390/nano11081900
Yadav, N., Chakraborty, B., Kumar, T.D.: First-principles study of a 2-dimensional C-silicyne monolayer as a promising anode in Na/K ion secondary batteries. Phys. Chem. Chem. Phys. 23(20), 11755–11763 (2021)
Jiang, H., Shyy, W., Liu, M., Wei, L., Wu, M., Zhao, T.: Boron phosphide monolayer as a potential anode material for alkali metal-based batteries. J Mater Chem A 5(2), 672–679 (2017). https://doi.org/10.1039/c6ta09264k
Lv, X., Li, F., Gong, J., Gu, J., Lin, S., Chen, Z.: Metallic FeSe monolayer as an anode material for Li and non-Li ion batteries: a DFT study. Phys. Chem. Chem. Phys. 22(16), 8902–8912 (2020). https://doi.org/10.1039/d0cp00967a
Anasori, B., Lukatskaya, M.R., Gogotsi, Y.: 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2(2), 1–17 (2017). https://doi.org/10.1038/natrevmats.2016.98
Winter, M.: J. 0. Besenhard, ME Spahr and P. Novak. Adv. Mater 10, 725–763 (1998)
Persson, K., Sethuraman, V.A., Hardwick, L.J., Hinuma, Y., Meng, Y.S., Van Der Ven, A., Ceder, G.: Lithium diffusion in graphitic carbon. J Phys Chem Lett 1(8), 1176–1180 (2010)
Lunell, S., Stashans, A., Ojamäe, L., Lindström, H., Hagfeldt, A.: Li and Na diffusion in TiO2 from quantum chemical theory versus electrochemical experiment. J. Am. Chem. Soc. 119(31), 7374–7380 (1997)
Olson, C.L., Nelson, J., Islam, M.S.: Defect chemistry, surface structures, and lithium insertion in anatase TiO2. J. Phys. Chem. B 110(20), 9995–10001 (2006)
Acknowledgements
This work was supported by the start-up funding at Chengdu University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Luo, Y., Wang, Z. et al. An Ideal Two-Dimensional Porous B4O2 as Anode Material for Enhancing Ion Storage Performance. Electron. Mater. Lett. 20, 275–282 (2024). https://doi.org/10.1007/s13391-023-00465-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13391-023-00465-w