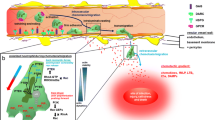
Abstract
The role and function of neutrophils are well known, but we still have incomplete understanding of the mechanisms by which neutrophils migrate from blood vessels to inflammatory sites. Neutrophil migration is a complex process that involves several distinct steps. To resist the blood flow and maintain their rolling, neutrophils employ tether and sling formation. They also polarize and form pseudopods and uropods, guided by hierarchical chemotactic agents that enable precise directional movement. Meanwhile, chemotactic agents secreted by neutrophils, such as CXCL1, CXCL8, LTB4, and C5a, can recruit more neutrophils and amplify their response. In the context of diapedesis neutrophils traverse the endothelial cells via two pathways: the transmigratory cup and the lateral border recycling department. These structures aid in overcoming the narrow pore size of the endothelial barrier, resulting in more efficient transmembrane migration. Interestingly, neutrophils exhibit a preference for the paracellular pathway over the transcellular pathway, likely due to the former’s lower resistance. In this review, we will delve into the intricate process of neutrophil migration by focusing on critical structures that underpins this process.


Similar content being viewed by others
References
Filippi, M. D. (2019). Neutrophil transendothelial migration: updates and new perspectives. Blood, 133(20), 2149–2158
Liew, P. X. & Kubes, P. (2019). The Neutrophil’s Role During Health and Disease. Physiological Reviews, 99(2), 1223–1248.
Mutua, V. & Gershwin, L. J. (2021). A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clinical Reviews in Allergy & Immunology, 61(2), 194–211.
Kolaczkowska, E. & Kubes, P. (2013). Neutrophil recruitment and function in health and inflammation. Nature Reviews Immunology, 13(3), 159–175.
Sundd, P., et al. (2010). Quantitative dynamic footprinting microscopy reveals mechanisms of neutrophil rolling. Nature Reviews Immunology, 7(10), 821–824.
Sundd, P., et al. (2012). ‘Slings’ enable neutrophil rolling at high shear. Nature, 488(7411), 399–403
Marmon, S., et al. (2009). Transcellular migration of neutrophils is a quantitatively significant pathway across dermal microvascular endothelial cells. Experimental Dermatology, 18(1), 88–90.
Carman, C. V. & Springer, T. A. (2004). A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. Journal of Cell Biology, 167(2), 377–388.
Springer, T. A. (1994). Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell, 76(2), 301–314
Heit, B., et al. (2002). An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. Journal of Cell Biology, 159(1), 91–102.
Phillipson, M. & Kubes, P. (2011). The neutrophil in vascular inflammation. Nature Medicine, 17(11), 1381–1390.
Ley, K., et al. (2007). Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews Immunology, 7(9), 678–689.
Petri, B., Phillipson, M. &Kubes, P. (2008). The physiology of leukocyte recruitment: an in vivo perspective. Journal of Immunology, 180(10), 6439–6446.
Zarbock, A., et al. (2011). Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood, 118(26), 6743–6751
Alon, R., et al. (1996). Interactions through L-selectin between leukocytes and adherent leukocytes nucleate rolling adhesions on selectins and VCAM-1 in shear flow. Journal of Cell Biology, 135(3), 849–865
Ivetic, A., Hoskins Green, H. L., & Hart, S. J. (2019). L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Frontiers in Immunology, 10, 1068
Shao, J. Y., Ting-Beall, H. P. & Hochmuth, R. M. (1998). Static and dynamic lengths of neutrophil microvilli. Proceedings of the National Academy of Sciences of the United States of America, 95(12), 6797–6802
Schmidtke, D. W., & Diamond, S. L. (2000). Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow. Journal of Cell Biology, 149(3), 719–730
Marki, A., et al. (2021). Elongated neutrophil-derived structures are blood-borne microparticles formed by rolling neutrophils during sepsis. Journal of Experimental Medicine, 218(3).
Wang, S., et al. (2018). S100A8/A9 in Inflammation. Frontiers in Immunology, 9, 1298
Xu, J., et al. (2003). Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell, 114(2), 201–214.
Yang, H. W., Collins, S. R., & Meyer, T. (2016). Locally excitable Cdc42 signals steer cells during chemotaxis. Nature Cell Biology, 18(2), 191–201
Bell, G. R. R., et al. (2021). Optogenetic control of receptors reveals distinct roles for actin- and Cdc42-dependent negative signals in chemotactic signal processing. Nature Communications, 12(1), 6148
McCormick, B., Chu, J. Y., & Vermeren, S. (2019). Cross-talk between Rho GTPases and PI3K in the neutrophil. Small GTPases, 10(3), 187–195
Hadjitheodorou, A., et al. (2021). Directional reorientation of migrating neutrophils is limited by suppression of receptor input signaling at the cell rear through myosin II activity. Nature Communications, 12(1), 6619.
Van Keymeulen, A., et al. (2006). To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. Journal of Cell Biology, 174(3), 437–445
Lam, P. Y., & Huttenlocher, A. (2013). Interstitial leukocyte migration in vivo. Current Opinion in Cell Biology, 25(5), 650–658
Houk, A. R., et al. (2012). Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell, 148(1-2), 175–188.
Szczur, K., Zheng, Y., & Filippi, M. D. (2009). The small Rho GTPase Cdc42 regulates neutrophil polarity via CD11b integrin signaling. Blood, 114(20), 4527–4537
Kumar, S., et al. (2012). Cdc42 regulates neutrophil migration via crosstalk between WASp, CD11b, and microtubules. Blood, 120(17), 3563–3574
Brunetti, R. M., et al. (2022). WASP integrates substrate topology and cell polarity to guide neutrophil migration. Journal of Cell Biol, 221(2), 1–23.
Lieber, A. D. et al. (2013). Membrane tension in rapidly moving cells is determined by cytoskeletal forces. Current Biology, 23(15), 1409–1417
Town, J. P., & Weiner, O. D. (2023). Local negative feedback of Rac activity at the leading edge underlies a pilot pseudopod-like program for amoeboid cell guidance. PLoS Biology, 21(9), e3002307
Gambardella, L., & Vermeren, S. (2013). Molecular players in neutrophil chemotaxis-focus on PI3K and small GTPases. Journal of Leukocyte Biology, 94(4), 603–612
Weiner, O. D. et al. (2002). A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nature of Cell Biology, 4(7), 509–513
Wang, F., et al. (2002). Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nature of Cell Biology, 4(7), 513–518
Kuiper, J. W., et al. (2011). Rac regulates PtdInsP3 signaling and the chemotactic compass through a redox-mediated feedback loop. Blood, 118(23), 6164–6171
Hirsch, E. et al. (2000). Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science, 287(5455), 1049–1053
Foxman, E. F., Campbell, J. J., & Butcher, E. C. (1997). Multistep navigation and the combinatorial control of leukocyte chemotaxis. Journal of Cell Biology, 139(5), 1349–1360
Phillipson, M., & Kubes, P. (2011). The neutrophil in vascular inflammation. Nature Medicine, 17(11), 1381–1390
Luster, A. D. (1998). Chemokines-chemotactic cytokines that mediate inflammation. The New England Journal of Medicine, 338(7), 436–445
Damaj, B. B. et al. (1996). Physical association of Gi2alpha with interleukin-8 receptors. Journal of Biology Chemistry, 271(22), 12783–12789
Rickert, P. et al. (2000). Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products. Trends in Cell Biology, 10(11), 466–473
Spisani, S. et al. (2005). A ‘pure’ chemoattractant formylpeptide analogue triggers a specific signalling pathway in human neutrophil chemotaxis. The FEBS Journal, 272(4), 883–891
Liu, X. et al. (2012). Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nature Reviews Immunology, 13(5), 457–464.
Mazaki, Y., et al. (2006). Neutrophil direction sensing and superoxide production linked by the GTPase-activating protein GIT2. Nature Immunology, 7(7), 724–731.
Subramanian, K. K., et al. (2007). Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood, 109(9), 4028–4037
Billadeau, D. D. (2008). PTEN gives neutrophils direction. Nature Immunology, 9(7), 716–718.
Chen, Y., et al. (2006). ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science, 314(5806), 1792–1795
Majumdar, R., Sixt, M. & Parent, C. A. (2014). New paradigms in the establishment and maintenance of gradients during directed cell migration. Current Opinion in Cell Biology, 30, 33–40.
Afonso, P. V., et al. (2012). LTB4 is a signal-relay molecule during neutrophil chemotaxis. Developmental Cell, 22(5), 1079–1091.
Tecchio, C. & Cassatella, M. A. (2016). Neutrophil-derived chemokines on the road to immunity. Seminars in Immunology, 28(2), 119–128.
Camous, L., et al. (2011). Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood, 117(4), 1340–1349
Sadik, C. D., et al. (2012). Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcγR signaling. Proceedings of the National Academy of Sciences of the United States of America, 109(46), E3177–E3185.
Subramanian, B. C., et al. (2022). The LTB4-BLT1 axis regulates actomyosin and β2-integrin dynamics during neutrophil extravasation. Journal of Cell Biology, 219(10), 1–14.
Majumdar, R., et al. (2021). Exosomes mediate LTB4 release during neutrophil chemotaxis. PLoS Biology, 19(7), e3001271
Phillipson, M., et al. (2006). Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. Journal of Experimental Medicine, 203(12), 2569–2575.
Jenne, C. N., Wong, C. H., Zemp, F. J., McDonald, B., Rahman, M. M., Forsyth, P. A., McFadden, G. & Kubes, P. (2013). Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe, 13(2), 169–180.
Carman, C. V., et al. (2007). Transcellular diapedesis is initiated by invasive podosomes. Immunity, 26(6), 784–797
Ridley, A. J., et al. (2003). Cell migration: integrating signals from front to back. Science, 302(5651), 1704–1709
Carman, C. V. (2009). Mechanisms for transcellular diapedesis: probing and pathfinding by ‘invadosome-like protrusions’. Journal of Cell Science, 122(Pt 17), 3025–3035.
Martinelli, R., et al. (2014). Probing the biomechanical contribution of the endothelium to lymphocyte migration: diapedesis by the path of least resistance. Journal of Cell Science, 127(Pt 17), 3720–3734.
Shaw, S. K., et al. (2004). Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. Journal of Experimental Medicine, 200(12), 1571–1580.
Barreiro, O., et al. (2005). Endothelial tetraspanin microdomains regulate leukocyte firm adhesion during extravasation. Blood, 105(7), 2852–2861
Singh, V. et al. (2023). ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clinica Chimica Acta, 548, 117487
Staunton, D. E., Dustin, M. L., & Springer, T. A. (1989). Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature, 339(6219), 61–64
Huang, M. T., et al. (2006). ICAM-2 mediates neutrophil transmigration in vivo: evidence for stimulus specificity and a role in PECAM-1-independent transmigration. Blood, 107(12), 4721–4727
Sun, J., et al. (2000). Contributions of the extracellular and cytoplasmic domains of platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) in regulating cell-cell localization. Journal of Cell Science, 113(Pt 8), 1459–1469.
Muller, W. A. et al. (1993). PECAM-1 is required for transendothelial migration of leukocytes. Journal of Experimental Medicine, 178(2), 449–460.
Takheaw, N., et al. (2019). Interaction of CD99 and its ligand upregulates IL-6 and TNF-α upon T cell activation. PLoS One, 14(5), e0217393
Gelin, C., et al. (1989). The E2 antigen, a 32 kd glycoprotein involved in T-cell adhesion processes, is the MIC2 gene product. The EMBO Journal, 8(11), 3253–3259.
Schenkel, A. R., et al. (2002). CD99 plays a major role in the migration of monocytes through endothelial junctions. Nature Immunology, 3(2), 143–150.
Watson, R. L., et al. (2015). Endothelial CD99 signals through soluble adenylyl cyclase and PKA to regulate leukocyte transendothelial migration. Journal of Experimental Medicine, 212(7), 1021–1041.
Martìn-Padura, I., et al (1998). Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. Journal of Cell Biology, 142(1), 117–127.
Johnson-Léger, C. A., et al. (2002). Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood, 100(7), 2479–2486
Ludwig, R. J., et al. (2005). Junctional adhesion molecules (JAM)-B and -C contribute to leukocyte extravasation to the skin and mediate cutaneous inflammation. Journal of Investigative Dermatology, 125(5), 969–976.
Bradfield, P. F., et al. (2007). JAM-C regulates unidirectional monocyte transendothelial migration in inflammation. Blood, 110(7), 2545–2555
Petri, B., et al. (2011). Endothelial LSP1 is involved in endothelial dome formation, minimizing vascular permeability changes during neutrophil transmigration in vivo. Blood, 117(3), 942–952
Sumagin, R., et al. (2014). Transmigrated neutrophils in the intestinal lumen engage ICAM-1 to regulate the epithelial barrier and neutrophil recruitment. Mucosal Immunology, 7(4), 905–915.
Liu, G., et al. (2012). ICAM-1-activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration. Blood, 120(9), 1942–1952
Allport, J. R., Muller, W. A. & Luscinskas, F. W. (2000). Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. Journal of Cell Biology, 148(1), 203–216.
Mamdouh, Z., et al. (2003). Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature, 421(6924), 748–753
Mamdouh, Z., Mikhailov, A. & Muller, W. A. (2009). Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. Journal of Experimental Medicine, 206(12), 2795–2808.
Sullivan, D. P., Seidman, M. A. & Muller, W. A. (2013). Poliovirus receptor (CD155) regulates a step in transendothelial migration between PECAM and CD99. The American Journal of Pathology, 182(3), 1031–1042.
Sullivan, D. P., & Muller, W. A. (2014). Neutrophil and monocyte recruitment by PECAM, CD99, and other molecules via the LBRC. Semin Immunopathol, 36(2), 193–209
Mamdouh, Z., Kreitzer, G. E. & Muller, W. A. (2008). Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. Journal of Experimental Medicine, 205(4), 951–966.
Sullivan, D. P., et al. (2019). Endothelial IQGAP1 regulates leukocyte transmigration by directing the LBRC to the site of diapedesis. Journal of Experimental Medicine, 216(11), 2582–2601.
Vestweber, D., et al. (2009). Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends in Cell Biology, 19(1), 8–15
Dejana, E., Orsenigo, F., & Lampugnani, M. G. (2008). The role of adherens junctions and VE-cadherin in the control of vascular permeability. Journal of Cell Science, 121(Pt 13), 2115–2122
Feng, G., et al. (2015). Segregation of VE-cadherin from the LBRC depends on the ectodomain sequence required for homophilic adhesion. Journal of Cell Science, 128(3), 576–588
Gonzalez, A. M., Cyrus, B. F., & Muller, W. A. (2016). Targeted Recycling of the Lateral Border Recycling Compartment Precedes Adherens Junction Dissociation during Transendothelial Migration. The American Journal of Pathology, 186(5), 1387–1402
Muller, W. A. (2016). Transendothelial migration: unifying principles from the endothelial perspective. Immunological Reviews, 273(1), 61–75
Vestweber, D. (2008). VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arteriosclerosis, Thrombosis, and Vascular Biology, 28(2), 223–232.
Wang, S., et al. (2022). Mechanosensation by endothelial PIEZO1 is required for leukocyte diapedesis. Blood, 140(3), 171–183
Arif, N., et al. (2021). PECAM-1 supports leukocyte diapedesis by tension-dependent dephosphorylation of VE-cadherin. The EMBO Journal, 40(9), e106113
Kenne, E., et al. (2019). Neutrophils engage the kallikrein-kinin system to open up the endothelial barrier in acute inflammation. The FASEB Jounal, 33(2), 2599–2609
Woodfin, A., et al. (2009). Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood, 113(24), 6246–6257
Lou, O., et al. (2007). CD99 is a key mediator of the transendothelial migration of neutrophils. Journal of Immunology, 178(2), 1136–1143
Arts, J. J., et al. (2021). Endothelial junctional membrane protrusions serve as hotspots for neutrophil transmigration. Elife, 10, e66074
Schaefer, A., et al. (2014). Actin-binding proteins differentially regulate endothelial cell stiffness, ICAM-1 function and neutrophil transmigration. Journal of Cell Science, 127(Pt 20), 4470–4482
Girbl, T., et al. (2018). Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity, 49(6), 1062–1076
Thiriot, A., et al. (2017). Differential DARC/ACKR1 expression distinguishes venular from non-venular endothelial cells in murine tissues. BMC Biology, 15(1), 45
Reglero-Real, N., et al. (2021). Autophagy modulates endothelial junctions to restrain neutrophil diapedesis during inflammation. Immunity, 54(9), 1989–2004
von Wedel-Parlow, M., et al. (2011). Neutrophils cross the BBB primarily on transcellular pathways: an in vitro study. Brain Research, 1367, 62–76
Wewer, C., et al. (2011). Transcellular migration of neutrophil granulocytes through the blood-cerebrospinal fluid barrier after infection with Streptococcus suis. Journal of Neuroinflammation, 8, 51
Millán, J., et al. (2006). Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nature Cell Biology, 8(2), 113–123
Dvorak, A. M. & Feng, D. (2001). The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. Journal of Histochemistry and Cytochemistry,49(4), 419–432
Liao, D., et al. (2022). Atomic Level Dissection of the Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1) Homophilic Binding Interface: Implications for Endothelial Cell Barrier Function. Arteriosclerosis, Thrombosis, and Vascular Biology, 42(2), 193–204
Acknowledgements
Thanks to all authors for their efforts in this article
Author information
Authors and Affiliations
Contributions
Z.W. and Y.G. wrote the original manuscript. B.W., Y.Z. and L.W. reviews and revised the original manuscript. Q.L. and L.W. prepared all the figures in this manuscript. All authors reviews the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have consented to the submission of the review to the journal. The authors declare no competing interest.
Consent for publication
All authors have consented to the submission of the review to the journal.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Guo, Y., Zhang, Y. et al. An Intriguing Structural Modification in Neutrophil Migration Across Blood Vessels to Inflammatory Sites: Progress in the Core Mechanisms. Cell Biochem Biophys 82, 67–75 (2024). https://doi.org/10.1007/s12013-023-01198-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-023-01198-1