Abstract
Global change drivers that modify the quality and quantity of litter inputs to soil affect greenhouse gas fluxes, and thereby constitute a feedback to climate change. Carbon cycling in the Yukon–Kuskokwim (Y–K) River Delta, a subarctic wetland system, is influenced by landscape variations in litter quality and quantity generated by herbivores (migratory birds) that create ‘grazing lawns’ of short stature, nitrogen-rich vegetation. To identify the mechanisms by which these changes in litter inputs affect soil carbon balance, we independently manipulated qualities and quantities of litter representative of levels found in the Y–K Delta in a fully factorial microcosm experiment. We measured CO2 fluxes from these microcosms weekly. To help us identify how litter inputs influenced greenhouse gas fluxes, we sequenced soil fungal and bacterial communities, and measured soil microbial biomass carbon, dissolved carbon, inorganic nitrogen, and enzyme activity. We found that positive correlations between litter input quantity and CO2 flux were dependent upon litter type, due to differences in litter stoichiometry and changes to the structure of decomposer communities, especially the soil fungi. These community shifts were particularly pronounced when litter was added in the form of herbivore feces, and in litter input treatments that induced nitrogen limitation (i.e., senesced litter). The sensitivity of carbon cycling to litter quality and quantity in this system demonstrates that herbivores can strongly impact greenhouse gas fluxes through their influence on plant growth and tissue chemistry.
Graphical abstract

Similar content being viewed by others
Introduction
The quality and quantity of litter inputs to soils affect soil carbon (C) balance and, thereby, feedbacks between the terrestrial C cycle and climate change. The chemical quality of litter [i.e., its ‘palatability’ to decomposers, which is often linked to lignin content and/or carbon:nitrogen (C:N) ratios] strongly influences rates of decomposition by imposing microbial nutrient and/or energy limitations (Cotrufo et al. 2013; Cleveland et al. 2014; Castellano et al. 2015). Meanwhile, quantity of litter inputs dictates the total amount of fresh organic matter available for decomposition, and it is the balance between plant C inputs and soil C losses through decomposer respiration that determines the size of the belowground C pool (Crow et al. 2009; Chemidlin Prévost-Bouré et al. 2010). Global change drivers impact litter quality and quantity, although these parameters may exhibit divergent responses to the same environmental perturbation. Thus, identifying and separating the effects of litter quality versus quantity impacts on belowground C cycling is key, because simultaneous shifts in litter traits may amplify or suppress one another’s effects (Castellano et al. 2015; Creamer et al. 2015). For example, increasing the magnitude of fresh soil C inputs can either protect (Blagodatskaya and Kuzyakov 2008) or destabilize (Liu et al. 2017; Shahbaz et al. 2017) native soil C stocks, with the direction and magnitude of response likely determined by C input chemistry.
There are several mechanisms by which litter input quality and quantity might interact to shape C cycling. Dissolved organic C (DOC), which is considered the most bioavailable soil C pool (Kalbitz and Kaiser 2008), is positively correlated with the quality of decomposing litter (Kalbitz et al. 2006; Klotzbücher et al. 2011). Thus, high quality litter inputs may have a disproportionate impact on soil C cycling because of an outsized increase in labile C availability compared to low quality litter, which could lead to greater soil microbial biomass (Fanin et al. 2014). Labile C inputs may induce large losses of soil C, even at relatively low amendment rates, by priming the decomposition of native soil C. Such positive priming effects generally occur when fresh inputs increase soil microbial biomass or stimulate the co-metabolism of ‘background’ soil organic matter (Blagodatskaya and Kuzyakov 2008). Indeed, positive priming effects have been found in N-poor subarctic soils due to the stimulation of “N-mining” in response to organic matter additions (Hicks et al. 2022; Na et al. 2022). Conversely, very high rates of labile C input can retard the decomposition of background C—a phenomenon known as ‘negative priming’—if microbes have a strong preference for the newly added substrate (Blagodatskaya and Kuzyakov 2008). Thus, the relationship between C input and soil C loss will depend upon the chemical composition and stoichiometry of new inputs in relation to microbial demand. In addition to priming effects, the relative concentrations of various plant compounds delivered to soil can affect total rates of decomposition by inducing or suppressing different extracellular enzyme activities. For example, microbial production of starch-degrading enzymes is not triggered until starch is present in relatively high concentrations, potentially inducing a non-linear relationship between the magnitude of C inputs and C losses (German et al. 2011a). Similarly, the production of energetically-costly lignin-degrading enzymes requires the presence of an easily available C source like DOC (Klotzbücher et al. 2011). As another example, if microbial production of extracellular enzymes is limited by the availability of N, even small amounts of N-rich litter input could trigger a burst of enzyme synthesis and thereby a dramatic acceleration of microbial decay (Schimel and Weintraub 2003).
Finally, soil microbial communities dictate the fate and rate of litter transformation (Schimel and Schaeffer 2012), so understanding how microbial communities respond to differences in litter quality and quantity may elucidate links between litter inputs and greenhouse gas fluxes. To this end, there have been decades-long efforts to group microbes into coherent ecological guilds with consistent effects on C cycling (Fierer et al. 2007; Malik et al. 2020; Morrissey et al. 2023). For example, the copiotroph-oligotroph framework predicts that copiotrophic microbes should predominate in the presence of high-quality substrates due to their capacity for rapid growth and generalist decomposition strategy, whereas oligotrophs should be more competitive on low-quality substrates (Fierer et al. 2007). Additionally, due to higher substrate use efficiency and resource scavenging ability, oligotrophs are expected to become more dominant when resource quantity is low (Ho et al. 2017). Therefore, this framework predicts that heterotrophic respiration per unit litter should be highest in scenarios of high-quality and high-quantity inputs due to the predominance of fast-growing, low-efficiency copiotrophs. In contrast, respiration per unit litter should be lowest under low-quality, low-quantity inputs when slow-growing, high-efficiency oligotrophs predominate.
Herbivory is an example of a process that simultaneously alters litter quality and quantity, likely having complex effects on C cycling. Herbivory often decreases aboveground litter input quantity via plant offtake, but subsequent quality impacts are unclear. Herbivory may trigger plant responses that lead to altered tissue N or lignin content (Wardle et al. 2002; Frost and Hunter 2008). The quality of plant litter may be further transformed via processing in the animal gut, because the plant remains re-deposited as feces are often enriched in inorganic nutrients (Petkuviene et al. 2019). Although herbivore-driven changes in litter quality and quantity often occur simultaneously in the field, current theoretical frameworks predict there could be important interactions between the two parameters (Grandy and Neff 2008; Manzoni et al. 2012; Castellano et al. 2015), necessitating a better understanding of their individual and combined effects.
Migratory geese in the coastal wetlands of the Yukon–Kuskokwim (Y–K) Delta, Alaska, affect both above- and belowground C cycling, altering soil greenhouse gas emissions through their simultaneous impacts on litter quality and quantity (Kelsey et al. 2016). In this ecosystem, vegetation is dominated by Carex spp. Geese, such as Branta bernicla negricans (Pacific black brant) and Branta hutchinsii (cackling geese), migrate to the Y–K Delta in the summer months and forage heavily to create swards of short-stature Carex known as ‘grazing lawns’. Grazed plant tissues have low C:N ratios and are an important source of nutritious forage for goslings (Ngai and Jefferies 2004; Sedinger et al. 2008). These grazing lawns also have high inputs of goose fecal matter (Beard et al. 2023). Nearby areas that are not heavily grazed have greater aboveground plant biomass, higher C:N ratios (Beard et al. 2019), and extensive accumulation of senesced Carex litter. Therefore, grazing increases litter nutrient content while simultaneously decreasing standing biomass, making the Y–K Delta an ideal system for examining how the quality and quantity of plant C inputs to soils jointly affect the fate of those inputs belowground.
The overall objective of this study was to determine how interactions between litter quality and quantity influence soil CO2 fluxes, using the Y–K Delta ecosystem as a case study. Specifically, we quantified: (1) the slope of the relationship between the proportion of total C utilized by decomposers (CO2–C flux per g C available) and the rate of C inputs, and (2) whether the sign or magnitude of these slopes depends upon the chemical characteristics (quality) of C inputs. We then assessed changes in soil nutrient availability, enzyme activities, and microbial communities to help us identify the underlying mechanisms behind litter quality-quantity interactions.
To do so, we conducted a fully factorial soil microcosm incubation experiment that separately manipulated the quality and quantity of soil C inputs. To capture variation in litter quality observed at our study site, we amended soils with inputs of varying chemical quality (as defined by litter N content and lignin:N ratios): grazed Carex; ungrazed, green (live) Carex; ungrazed, senesced Carex; and goose feces (which consists mainly of digested plant material, likely Carex). Each litter type was added at one of three rates spanning an order of magnitude which represent the range of annual litter inputs we expect that the Y–K Delta receives. We anticipated a positive relationship between the proportion of C respired and C input quantity, in line with previous studies (e.g., Shahbaz et al. 2017). However, we expected this correlation to be strongest in soils amended with higher-quality litters because: (H1) Increasing rates of N-rich, high-quality litter addition should progressively relieve microbial N limitation and increase C bioavailability (as DOC). This should support more microbial biomass and stimulate C- and N-degrading enzyme activities, inducing priming effects. By contrast, low-quality litters should exacerbate restrictions on microbial growth. We also expected that (H2) high inputs of high-quality litter should induce a shift to copiotrophic-dominant microbial communities with elevated rates of respiration.
Methods
Study site
We collected soils and litter for this study in the Yukon Delta National Wildlife Refuge along the Kashunuk River (61°19′33″ N, 165°29′48″ W, approximately 4 km from the coast). The climate is maritime, with 30-year mean (1991–2020) winter daily temperatures of − 12.2 °C and mean summer daily temperatures of 12.5 °C. Thirty-year mean annual precipitation is 499 mm (rain + snow-water equivalent), and mean annual snowfall is 1626 mm (Palecki et al. 2021). In the winter, the ground is typically covered with snow, and the heaviest rains occur from July to September (Tande and Jennings 1986). Soils are predominantly silt and sand deposits, with pH between 6.8 and 7.0 (Tande and Jennings 1986; Foley et al. 2021). Carex subspathacea is the dominant vegetation and forms extensive wet-sedge meadows with an average vegetation height of ~ 15 cm (Kelsey et al. 2016). Grazing of C. subspathacea by Pacific black brant and cackling geese results in ‘grazing lawns’ in which the C. subspathacea is converted to a short-statured form of ~ 1 cm (Person et al. 2003; Kelsey et al. 2016).
Field collections and processing
On 4 August 2021, we collected 18 blocks of soil along a 100 m transect in an ungrazed C. subspathacea meadow. Every 20 m along the transect, we collected three 10 × 10 cm soil blocks to a depth of 15 cm using a sterile knife from the north, east, and south cardinal directions at 1 m from the transect. We collected soils from ungrazed meadows rather than grazed areas because ungrazed meadow constitutes the majority of vegetated surface area in this part of the Y–K Delta (Person et al. 2003), so soils from ungrazed meadows are most representative of the ecosystem. Additionally, because geese convert ungrazed meadow to grazing lawn over a period of several years (Person et al. 2003), soil from ungrazed meadows represent “time zero” conditions before litter quality and quantity and microbial communities are altered due to grazing pressure.
We also collected ungrazed green (live; ≥ ~15 cm height) C. subspathacea and senesced C. subspathacea from ungrazed meadows near the transect, and we collected grazed C. subspathacea (short < 1 cm height) and goose feces from grazing lawns near the transect. We considered the tall-form C. subspathacea outside of grazing lawns to be ungrazed, but we cannot be absolutely certain these plants had never experienced herbivory. Nonetheless, we did not observe any evidence of recent grazing (e.g. trampling, fecal deposition) outside of the grazing lawns.
We transported soils to Utah State University on ice. Within 48 h of collection, we subsampled a 1 × 1 × 15 cm portion of each block and stored them in a − 80 °C freezer for microbial community analyses. We oven-dried and homogenized litter types by grinding them, and then measured total C and N content on an elemental analyzer (ECS4010 Elemental Analyzer, Costech Analytical Technologies, Valencia, CA, USA). Phosphorus and potassium content were measured by the Utah State University Analytical Laboratories via near-infrared spectroscopy (NIRS DS2500 F, FOSS Analytics, Hillerød, Denmark). We analyzed lignin using acid detergent fiber methods (Gessner 2005).
Experimental design
To determine the effects of litter quality and quantity on soil C cycling, we performed a 12-week soil microcosm experiment, representing the length of the growing season on the Y–K Delta. The microcosm experiment consisted of a fully factorial manipulation of four litter quality treatments (grazed C. subspathacea, ungrazed green (live) C. subspathacea, goose feces, and senesced C. subspathacea) added at three C input quantity levels (0.4 mg C g−1 soil, 2.4 mg C g−1 soil, 4.7 mg C g−1 soil, equivalent to 2.4, 14.4, and 28 g m−2; hereafter low, medium, and high). To ensure C input rates ranged within the bounds observed in this ecosystem, we chose our treatment levels based upon previous measurements of aboveground biomass of three representative vegetation communities in this part of the Y–K Delta. The three reference vegetation communities (slough levee, ungrazed wet sedge meadow, and grazed wet sedge meadow) are all graminoid-dominated and have been described in detail elsewhere (Jorgenson 2000; Kelsey et al. 2016). The three reference communities represent a gradient of standing aboveground biomass, with grazed meadows having the lowest biomass, ungrazed meadows having intermediate quantities, and slough levees having the highest biomass. We converted the standing aboveground biomass quantities of these three communities (data from Kelsey et al. 2016) to litter C inputs, assuming that 10% of litter in each habitat is incorporated into the soil per year, and that ~ 40% of the plant biomass is C. The assumption of 10% litter incorporation is a conservative estimate based on our personal observations that the majority of senesced biomass remains standing aboveground through future growing seasons. In a separate experiment, we found that the ratio of standing senesced biomass to live biomass in ungrazed Carex meadow was ~ 1.13 at peak growing season (unpublished data). This indicates that most of the biomass that grew in previous years was still standing aboveground and was not yet incorporating into the soil.
The C:N ratios, lignin, and nutrient analyses of the litter informed the litter quality treatments (Table 1). We adjusted the mass of litter added within each quantity treatment to reflect the variation in C content of each litter type; therefore, all experimental units in the same litter quantity treatment received identical amounts of C, regardless of litter type. This design resulted in 12 unique treatment combinations with 10 replicates each; we also included 10 control soils with no litter additions to help us assess priming effects (see Sect. 4.1). Note that not all of our treatments are intended to represent actual (or potential) C input regimes at the site—for example, it is highly unlikely that 4.7 mg C cm−3 of feces would ever be delivered to the soil in the Yukon Delta. However, this experimental design did make it possible to directly compare slopes of the CO2 flux-C input relationship across the different litter types.
Each microcosm consisted of 30 g dry weight of homogenized soil in a 250 mL glass jar fitted with a septum. We adjusted soil moisture content in microcosms to 60% of water holding capacity, which maximizes soil heterotrophic respiration (Zhou et al. 2014). At the beginning of the experiment, we adjusted microcosms for moisture content using a solution with a salinity of 28 g L−1 total dissolved sodium salts (Instant Ocean SeaSalt, Spectrum Brands, Blacksburg, VA, USA) (Person and Ruess 2003) to represent the brackish water these soils are typically exposed to. We weighed microcosms weekly to determine water loss and maintained soil moisture levels by adding deionized water when necessary (to avoid continuous increases in soil salt content). We incubated microcosms in the dark at 10 °C to simulate mean temperatures during the growing season in the field (Jorgenson and Ely 2001).
Laboratory analyses
Once per week over the incubation period, we sealed the microcosms and allowed gas to accumulate in the headspace over 24 h. We used gas chromatography to analyze concentrations (µmol) of carbon dioxide (CO2) that accumulated in the headspace (GC-2014 Greenhouse Gas Analyzer, Shimadzu, Kyoto, Japan; 8610 C Gas Chromatograph, SRI Instruments, Las Vegas, Nevada). At the start of the experiment and following each headspace sampling, we randomized microcosm locations within the incubator.
At the end of the incubation, we stored soil subsamples from each microcosm at − 80 °C for analysis of enzyme activity and microbial DNA. In addition, we determined total inorganic nitrogen (TIN) as the sum of ammonium (NH4) and nitrate (NO3) N content in each microcosm, measured through colorimetric analysis of 2 M KCl extracts.
Microbial analyses
At the conclusion of the incubation, we measured microbial biomass in each microcosm using the direct chloroform fumigation and extraction method (Witt et al. 2000), and analyzed the resulting extracts for total dissolved C content on a TOC-L (Shimadzu, Kyoto, Japan). We determined microbial biomass carbon (MBC) as the difference between C content in K2SO4 fumigated and unfumigated soil extracts. We determined extracellular enzyme activities of cellobiase (CB), N-acetylglucosaminidase (NAG), leucine aminopeptidase (LAP), acid phosphatase (AP), and β-glucosidase (BG) with p-nitrophenol linked substrates (German et al. 2011b).
Following manufacturer protocols, we extracted DNA from each of the 130 microcosm soil samples and five subsamples of pre-incubation soils using a DNeasy PowerSoil Kit (Qiagen, Hilden, Germany). DNA extracts were pooled in equimolar concentrations and pair-ended sequenced (2 × 250) using the Illumina MiSeq platform at the USU Center for Integrated Biosystems. Following Earth Microbiome project protocols, 16 S-rRNA regions were amplified with 515 F-806R primers, and ITS regions were amplified using ITS1f-ITS2 primers (Caporaso et al. 2011, 2012; Thompson et al. 2017). We used the QIIME 2 bioinformatics pipeline to process and assign taxonomy to soil bacterial and fungi communities (Bolyen et al. 2019). We determined amplicon sequence variants (ASVs) using the DADA2 algorithm (Callahan et al. 2016). We separated taxonomic units of bacterial and fungal sequences using the Greengenes database to train a Naïve Bayesian classifier (DeSantis et al. 2006). We identified fungal groups using the UNITE database (Nilsson et al. 2019) in combination with the BLAST + algorithm (Camacho et al. 2009). We rarefied each sample for a total of 25,000 sequences for fungal communities and 40,000 sequences for bacterial communities. We used the rrnDB database (Stoddard et al. 2015) to predict rrn gene copy number for each bacterial ASV according to the method of (Li et al. 2021), and we calculated community-weighted mean abundance of rrn gene copies for each soil sample following the approach of (Nemergut et al. 2016). Community-weighted rrn copy number is a community-aggregated indicator of bacterial ecological strategies, where higher rrn copy number indicates a more copiotrophic lifestyle (Klappenbach et al. 2000; Fierer et al. 2007).
Statistical analyses
We used linear mixed-effects models to determine the effects of litter input quantity (in g C), quality (litter type), incubation day and their interactions on CO2 fluxes, and included microcosm identifiers as a random effect to control for the non-independence of CO2 measurements taken from the same microcosm over time. All C fluxes were standardized to the total amount of C available in each microcosm, including litter inputs (i.e., flux units were µg CO2–C g−1 C h−1). In effect, this means that we tested whether the proportion of soil/litter C that is utilized increases with the total quantity of C available. To further assess how different litter input treatments elevated soil respiration rates against the ‘background rate’ of soil respiration, we performed separate mixed effects models to assess treatment effects relative to no-litter controls. These models included a fixed effect for litter treatment (quantity or quality), incubation day, and a random effect for microcosm identity. Finally, we note that treatment-related differences in respiration rates would cause the ‘bioavailable’ C pool to exhibit different temporal dynamics in each treatment group. Therefore, we also employed a first-order exponential decay model to explore how the litter quality and quantity treatments affected respiration after controlling for C availability (Kuzyakov 2011).
To determine whether priming effects (i.e. enhanced decomposition of pre-existing soil C with litter additions) impacted the CO2 fluxes we observed, we calculated a conservative estimate of priming effect as follows (Blagodatskaya and Kuzyakov 2008):
where Ctreatment is the cumulative C respired in a microcosm receiving litter amendments, Ccontrol is the cumulative C respired in the microcosms receiving no litter inputs, and Clitter is the quantity of litter C added.
We used general linear models to analyze post-incubation measurements, including cumulative CO2 fluxes, microbial biomass, dissolved organic C, inorganic–N pools, bacterial weighted-mean rrn copy number, and enzyme activities. All models included litter quality and quantity as main effects, and their interaction. We removed outliers greater than 3.5 standardized residual variances above the mean. When significant main effects of a treatment were found, we performed post-hoc pairwise comparisons with a Tukey adjustment. We also determined the relative contribution of quality, quantity, and their interaction to the model R2 value using the lmg metric with the R package “relaimpo” (Groemping and Matthias 2021).
All statistical analyses were performed in the R statistical computing environment (R Core Team 2023). Linear and non-linear mixed models were performed in the R package “nlme” (Pinheiro et al. 2019). General linear models were performed using the lm command in base R, and post-hoc comparisons were performed in the R package “emmeans” (Lenth 2020).
We determined variation in microbial communities among treatment types by performing non-metric multidimensional scaling (NMDS) and Permutational Analysis of Variance (PERMANOVA) on Bray-Curtis distance matrices using the R package “vegan” (Oksanen et al. 2019). We followed the recommendations of Nearing et al. (2022) to determine which microbial taxa contributed to community differences among treatments by performing differential abundance analysis using four separate differential abundance tools. We used the R packages “ANCOMBC” (Lin and Peddada 2020), “Maaslin2” (Mallick et al. 2021), “ALDEx2” (Fernandes et al. 2013), and “microbiomeStat” (Zhou et al. 2022) for these analyses. We considered a taxon differentially abundant if at least three of the tools identified it as such. We performed post-hoc pairwise comparisons for taxa that were significantly affected by treatments using Benjamini–Hochberg adjusted Wilcoxon rank sum tests on centered log-ratio (clr) transformed abundance data. The clr transformation is the log-ratio of abundance of a single taxon to the mean abundance of all taxa in a sample. A positive clr value therefore indicates that the taxon is more abundant than average, and a negative clr value indicates the taxon has less than average abundance.
Results
CO2 fluxes
Litter quality and quantity interacted to affect soil respiration (Fig. 1; Table 2). CO2 fluxes (expressed per g C available in each microcosm) always increased with C input quantity: on average, microcosms in the high-quantity and medium-quantity litter treatments produced CO2 fluxes 75 and 35% higher than microcosms in the low-quantity treatments, respectively (Fig. 2). However, the positive influence of C input quantity on respiration was most pronounced when C was added in the form of grazed Carex or feces, and least evident when C was added as ungrazed, senesced Carex (Fig. 1). Moreover, litter quality and quantity effects on CO2 flux varied with time (Fig. 2; Table 2). Microcosms amended with the largest amounts of grazed litter exhibited the highest CO2 fluxes through day 36 of the experiment; thereafter, microcosms amended with feces exhibited the highest respiration rates (Figs. 2, S1). As a result, cumulative CO2 flux across the 12-week incubation was greatest in microcosms receiving the highest quantity of C inputs, in the form of feces (Tables S1, S2). Cumulative fluxes were smallest in microcosms receiving the lowest quantity of C inputs, regardless of the quality of litter added. In fact, in post-hoc tests, CO2 fluxes in microcosms receiving the lowest rates of litter addition were not significantly different from no-input controls. These patterns were robust even after accounting for differences in the bioavailable C pool’s depletion rate using an exponential decay model (Table S4). Overall, our linear model predicted 94.3% of the variation in cumulative respiration, with 72.3% explained by the quantity treatment, 14.4% by the quality treatment, and 7.5% by their interaction.
The relationship between C input rate (litter quantity) and soil respiration for microcosms in each litter quality treatment: grazed Carex sampled from C. subspathacea grazing lawns, ungrazed live (green) Carex sampled outside the grazing lawns, goose feces, and senesced Carex, also sampled outside the grazing lawns. Slopes and intercepts are taken from a linear mixed effects model performed on weekly CO2 fluxes, standardized to the total quantity of litter and soil C available in each microcosm
Mean cumulative CO2 fluxes (± SE) for treatment types a by litter quality and b by litter quantity. Microcosms with no additions were not included in linear models for cumulative fluxes, but are included in the figures for comparison. These values are standardized to the g C contained in each microcosm, inclusive of the C added from treatments
For all microcosms, the calculated priming effect was negative, and litter quality and quantity interacted to influence the priming effect (p < 0.001). There were no significant differences in priming among litter types within the low quantity treatments. Within the medium quantity treatments, the magnitude of the priming effect mirrored the respiration response, following the order: feces > grazing lawn = green live > senesced litter; the priming effect within high quantity treatments also mirrored respiration response, following the order: feces > grazing lawn > green live > senesced litter (data not shown).
Soil biogeochemistry
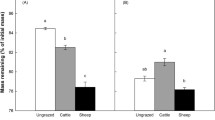
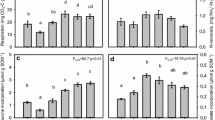
Litter quality, quantity, and their interaction all significantly affected soil TIN after the 12-week incubation (Fig. 3a; Table S1). Averaged across all litter types, high-quantity treatments resulted in 18% higher TIN than low-quantity treatments, but the pattern was more pronounced for N-rich litters. In general, soil TIN concentrations across the litter quality treatments reflected the N content of each litter type. The largest amounts of inorganic N accumulated in microcosms amended with N-rich grazed Carex, whereas TIN concentrations were lowest—indeed, 20% lower than in no-input control treatment—in microcosms amended with N-poor senesced Carex.
Mean (± SE) a Total Inorganic Nitrogen, b Microbial Biomass Carbon, c Dissolved Organic Carbon by quality treatment, and d Dissolved Organic Carbon by quantity treatment. Microcosms with no additions were not included in linear models. “N.A.” are no-addition, “G. live” are green live, and “G. lawn” are grazing lawn treatments
Neither litter quality nor quantity treatments significantly affected MBC after 12 weeks of incubation (Fig. 3b; Table S1). However, compared to no-addition controls, medium- and high-quantity litter addition treatments produced 20–24% higher MBC, and soils amended with litter from grazing lawn treatments produced 25% higher MBC than microcosms with no C inputs (Fig. 3b; Table S3). Meanwhile, DOC responded to the independent effects of litter quality and quantity (Fig. 3c, d; Table S1). High-quantity and medium-quantity litter additions resulted in 29% and 21% more DOC than low-quantity additions, respectively, and feces additions resulted in 15–25% more DOC than other litter types. All litter treatments elevated DOC concentrations above those seen in microcosms without C inputs (Table S3). Finally, NAG was the only enzyme to respond to the litter input treatments: activity was 18–22% greater in the low- and medium-quantity litter addition treatments compared to high-quantity additions (p = 0.03 and p = 0.01, respectively) (Fig. S2).
Microbial communities
Litter quality and quantity interacted to affect bacterial community-weighted mean rrn copy number (p < 0.001; Fig. S3). High quantity treatments tended to result in higher weighted mean rrn copy numbers than low quantity treatments, except within the senesced litter treatments, where quantity did not affect copy number. The impacts of litter quality on mean rrn copy number varied slightly with quantity. Within medium and high quantity treatments, feces and green live additions had the highest weighted mean copy numbers; within the low quantity treatments, feces and senesced litter additions had higher weighted mean copy numbers than grazed Carex additions.
The quality and quantity of litter inputs significantly interacted to affect microbial community structure (Figs. 4 and 5), although litter quality explained more of the variance in bacterial and fungal community structure than litter quantity. Differential abundance analysis indicated that litter quality and quantity interacted to affect the abundance of nine bacteria phyla (Figs. S4–S12). Among the most abundant bacteria, Firmicutes and Bacteroidetes exhibited the strongest treatment responses as indicated by large differences in clr transformed abundance. Within the medium- and high-quantity treatments, differences in Firmicutes abundance were primarily driven by senesced litter additions, which resulted in Firmicutes abundance that was 0.09–0.58 clr units lower than other litter types. Within the low-quantity treatment, Firmicutes abundance in the senesced treatment was 0.48 clr units higher than in the grazing lawn treatment. Differences in Bacteroidetes abundance were driven by senesced litter and feces. Feces additions increased Bacteroidetes abundance within the medium and high quantity treatments. Senesced litter additions decreased Bacteroidetes abundance within the medium quantity treatments. In addition to responding to litter treatments, post-incubation bacterial communities differed markedly from field-fresh pre-incubation communities (Fig. 5a). Firmicutes abundance exhibited the largest change, increasing from a clr abundance of 1.36 in field-fresh soils to 6.63 in post-incubated no-addition soils. Post-incubation Bacteroidetes and Planctomycetes abundance also increased, whereas Acidobacteria and WS3 abundance decreased.
a Non-metric multidimensional scaling plots conducted on Bray–Curtis distance matrices for bacterial (stress = 0.12; Quality p = 0.001, R2 = 0.310; Quantity p = 0.001, R2 = 0.076; Quality × Quantity p = 0.001, R2 = 0.105) and b fungal communities (stress = 0.07; Quality p = 0.001, R2 = 0.283; Quantity p = 0.001, R2 = 0.038, Quality × Quantity p = 0.001, R2 = 0.098) based on treatments. Microcosms with no additions were not included in PERMANOVA analysis. Point size denotes different quantity treatments, with larger dots corresponding to
higher quantity additions
Relative abundances of a bacterial phyla and b fungal classes. Groups marked as “other” denote classes or phyla that were less than 1% abundant. Groups marked as “unidentified” were not identified to the class level. “Pre-Inc” refers to pre-incubation soils; “N.A.” are no-addition, “G. live” are green live, and “G. lawn” are grazing lawn treatments
Differential abundance analysis indicated that litter quality and quantity also interacted to affect the abundance of seven fungal classes (Figs. S13–S19). Pezizomycetes, Dothideomycetes, and Tremellomycetes exhibited the strongest treatment responses. The responses to quality treatments for these classes tended to be strongest within the high- and medium-quantity treatments. Pezizomycetes clr abundance in feces treatments increased to 6.61 and 5.91 within the medium and high quantity treatments, respectively, compared to an average of − 0.26 for the other litter types. Dothideomycetes clr abundance in grazing lawn treatments was 1.37–3.42 units higher than other litter types within the medium- and high-quantity treatments. Within the medium- and high-quantity treatments, Tremellomycetes abundance was higher in the grazing lawn and green live treatments than in the feces and senesced treatments. Similar to bacteria, post-incubation fungal communities differed from field-fresh pre-incubation communities irrespective of litter treatments (Fig. 5b). Specifically, fungal communities shifted from predominately unidentified fungal classes in pre-incubated soils to a composition with substantial representations from Saccharomycetes, Dothideomycetes, Sordariomycetes, and Eurotiomycetes classes in post-incubated, no-litter addition soils.
Discussion
Our results suggest that both the quality and quantity of litter are important determinants of litter decomposition. In line with our expectations, higher quantities of litter addition resulted in higher cumulative CO2 efflux per unit C added, as found in other studies (Liu et al. 2017; Shahbaz et al. 2017). This phenomenon may be attributable to ‘hotspots’ of organic matter availability in soils under high rates of litter amendment, which alleviate spatial constraints on microbial litter access (Schnecker et al. 2019). However, the degree to which elevated C input stimulated CO2 flux depended on the type of litter added. This result is important, as it suggests that the effects of increased plant productivity on soil C balance will hinge on the chemistry of those plant C inputs. The mechanisms underlying this pattern may relate to differences in the chemical recalcitrance or stoichiometry of the plant inputs; additionally, litter quantity–quality interactions may emerge from shifts within decomposer microbe communities. Below, we consider support for each of our hypotheses in turn.
Effects of litter quality on respiration across a gradient of litter input quantity: the role of plant tissue chemistry
We expected soil respiration to correlate more strongly with C input rate in ‘high-quality’ (high-N, low lignin:N) litter treatments, because these inputs should alleviate N limitation and allow for increased decomposition of both fresh and native soil organic matter (Blagodatskaya and Kuzyakov 2008). In fact, microcosms amended with senesced Carex—which exhibited the lowest rates of respiration—had less TIN than control soils with no litter inputs, indicating net N immobilization and (potentially) aggravation of N limitation. Additionally, we found that the activity of the extracellular enzyme NAG—which targets N-rich chitin—was suppressed when litter was added at high rates, suggesting high quantity additions alleviated the need to invest in N-acquisition.
However, N cycle parameters did not perfectly correlate with patterns of CO2 flux across the different treatments. Concentrations of TIN at the end of the incubation paralleled the N content of litter inputs, but cumulative CO2 flux did not follow the same pattern, suggesting that litter characteristics other than N-content were most important for dictating the respiration response. For example, cumulative respiration rates were highest in microcosms amended with feces, even though this type of litter input had a relatively low N content, a high lignin:N ratio, and somewhat smaller TIN pools at the conclusion of the incubation. Instead, other limiting factors, such as the amounts of bioavailable C or inorganic nutrients could have affected decomposition dynamics. For example, the high CO2 flux from feces-amended microcosms could be due to the greater C availability (suggested by high DOC), which could have fueled lignin degradation (Klotzbücher et al. 2011). Additionally, high respiration in microcosms receiving feces could have been driven by P availability, as feces had the lowest C:P ratio of all litters by a large margin. Overall, we were able to demonstrate partial support for our hypothesis that the N content of litter influences its decomposition along gradients of C availability; however, this was primarily evident when litter quality was very low (i.e. high lignin:N and C:N). In the case of higher quality litters, it appears that other limiting resources (labile C, P) play an outsized role in dictating decomposition.
To what extent did priming effects contribute to the patterns we observed? Because we standardized respiration rates to the quantity of C added to each microcosm, our findings reflect greater C utilization with higher rates of C input, especially for high-quality litter. This could represent ‘true’ priming effects (enhanced decomposition of pre-existing soil C), or ‘apparent’ priming (more complete decomposition of the added litter), so we calculated priming effect to distinguish between these phenomena. For all microcosms, priming effect values were negative, indicating that the magnitude of C inputs exceeded litter-induced C losses (i.e., all soils accumulated C throughout the incubation). Without isotopic labelling, we cannot precisely quantify the source of C respired in each treatment (background soil organic matter vs. new litter input). However, ‘true’ priming effects—if they occurred—were not large enough to trigger a net soil C loss, in any litter quantity or quality treatment. Despite this, it is clear that the total magnitude of C loss from each individual microcosm was dependent on both the amount and chemical composition of C added.
Effects of litter quality on respiration across a gradient of litter input quantity: the role of microbial community shifts
Because the size of the microbial biomass did not vary among the different litter quantity or quality treatments, changes in microbial C use efficiency or biomass turnover rate—both of which could be associated with community shifts—likely drove the changes in C cycling we observed (Fierer et al. 2007; Malik et al. 2020; Morrissey et al. 2023). We found evidence in support of our hypothesis that greater C respiration was associated with more copiotrophic microbial communities. Specifically, we found that mean weighted rrn copy number of bacterial communities was highest for feces addition treatments and tended to increase with litter quantity. rrn copy number has been shown to associate with putative copiotrophic traits like high maximum growth rate and low C use efficiency (Klappenbach et al. 2000; Roller et al. 2016), and copiotrophic microbes are thought to exhibit faster biomass turnover rates (Fierer et al. 2007), all of which could have contributed to the higher respiration rates in the feces amended soils. Contrary to our hypothesis, however, the litters with the lowest lignin:N ratios did not result in the most copiotrophic bacterial communities, indicating that community shifts toward copiotrophy were driven by something other than litter N and lignin. In fact, community weighted rrn copy number was positively correlated with soil DOC at the end of the experiment (p = 0.001, r = 0.28). This could indicate that greater C availability promoted more copiotrophic bacterial communities, although it is also possible that higher DOC concentrations resulted from faster biomass turnover rates in these communities.
It is important to note that community-weighted rrn copy number does not consider fungal communities, which also contribute to the degree of copiotrophy vs. oligotrophy within soils (Ho et al. 2017). In fact, fungal communities exhibited more dramatic compositional shifts to our litter treatments than bacterial communities. These strong fungal responses may suggest that fungi were more functionally distinct than bacteria in this system, and therefore potentially more important for driving differences in decomposition among treatments. In contrast, bacterial communities differentiated more strongly from pre-incubated soils than they did among litter addition treatments. This indicates that bacterial community turnover was primarily driven by successional dynamics. However, as we did not directly manipulate microbial communities, we cannot say definitively whether fungi or bacteria played a more important role in driving differences in litter decomposition.
The most conspicuous effects of litter quality on fungal communities occurred within the feces treatments, which became strongly dominated by the Pezizomycetes fungal class, and the grazing lawn treatments, which became strongly dominated by the order Pleosporales from the Dothideomycetes class. Pezizomycetes are well-documented dung-associated fungi (Pfister and Healy 2021), and Pleosporales have previously been observed to strongly predominate during the decomposition of a Carex species (Ma et al. 2020). Therefore, the fast decomposition rates in the feces and grazing lawn treatments, especially under the highest rates of litter amendment, could be due to the development of functionally distinct fungal communities that were well matched to the available litter type.
In general, microbial communities differentiated across the litter quality treatments only at medium and high levels of C input, paralleling trends observed for respiration. One notable exception to this pattern was observed for fungal communities receiving low inputs of senesced litter: these strongly differed from the assemblages found in all other treatments. Perhaps this is a signal of the accentuated N limitation in the senesced litter treatment. Moreover, we note that the pre-incubation microbial community composition differed greatly from all post-incubation microbial communities, including those not amended with litter. Therefore, although we cannot directly extrapolate our data from the experimental microcosms to the field, our findings strongly suggest that differences in litter quality have the potential to affect C utilization patterns via their effects on bacterial and fungal communities.
Implications for C cycling on the Y–K delta
Plants influence soil C cycling through the quantity and chemical composition of their litter inputs, and herbivores can strongly impact both traits. Here we show that three known effects of herbivory can modify greenhouse gas fluxes from wetland soils through multiple mechanisms (1) by dramatically reducing the quantity of aboveground plant tissue available for decomposition, (2) by enhancing the nutrient content of vegetation, (3) by further modifying litter chemistry before re-deposition as feces. Each of these changes, in turn, was associated with significant shifts in the composition of soil microbial communities. Thus, a complex interaction among animals, plants, bacteria, and fungi ultimately governs ecosystem carbon balance in this high-latitude wetland.
Herbivores strongly influence both litter quality and quantity on the Y–K Delta, with opposing effects on overall CO2 flux. Our results suggest that herbivores—which create grazing lawns and feces—accelerate soil organic matter cycling by altering litter quality. At the same time, by reducing C inputs, herbivores may strongly suppress soil C fluxes. Which effect predominates in situ? Field studies in the Y–K Delta imply that the latter effect may play a stronger role in determining overall soil C balance: field-measured ecosystem respiration is higher in ungrazed habitats than grazed habitats (Kelsey et al. 2016), likely because of the much larger inputs of litter in ungrazed areas. However, field-measured ecosystem respiration in the ungrazed Carex meadows is only ~ 1.5 times greater than in grazing lawns, despite litter inputs being up to six-fold greater (Kelsey et al. 2016). This suggests that soil C accumulation is higher in ungrazed meadows, and that goose herbivory decreases the strength of the ecosystem C sink by reducing litter quantity.
Additionally, grazing-induced alterations in litter type and quantity are both associated with changes in microbial communities, providing further context for previously observed differences in microbial communities between grazed and ungrazed meadows in the Y–K Delta (Foley et al. 2021). In that study, grazing lawn soils incubated without external C amendments exhibited higher respiration rates than ungrazed soils, suggesting the soil microbes associated with grazing lawns have an inherently higher rate of metabolism, and that more labile C is found in grazing lawn soils, perhaps due to enhanced root exudation (Sun et al. 2017). This indicates that microbial communities in grazing lawns are more copiotrophic than in ungrazed soils, a pattern that our findings suggest may be partially driven by feces deposition favoring copiotrophic bacteria. This effect may explain why field-measured ecosystem respiration in grazing lawns is so much higher than would be expected based on the quantity of litter inputs relative to ungrazed Carex meadows (Kelsey et al. 2016). In addition to impacting soil CO2 flux, more copiotrophic communities in grazing lawns may also influence soil C persistence. The lower C use efficiency of copiotrophic communities leads to less accumulation of microbial necromass, which may be preferentially stabilized in soil compared to plant-derived inputs (Liang et al. 2017, 2019). Determining how grazing influences soil C persistence in sub-arctic and arctic ecosystems is an important avenue for future research.
This study can also inform our understanding of how CO2 fluxes may change in this system with potential shifts in vegetation quality and quantity, due to changes in herbivore distributions. Black brant populations in the Y–K Delta have been experiencing declines over several decades (Fondell et al. 2011), and our results suggest that the subsequent decline in grazing meadows could alter the C source strength of the region (Leffler et al. 2019). In our experiment, heterotrophic respiration in treatments typical of ungrazed meadows (high quantity, senesced and green live Carex) was ~ 50% higher than in treatments typical of grazed meadows (low quantity, grazed Carex and goose feces). Our experiment also shows a surprising effect of herbivory on microbial communities, with unique fungal communities associated with feces addition, alongside unexpectedly high respiration rates. Similar effects on soil microbial communities could occur in other systems with high densities of animals, with consequences for soil functioning and climate change feedbacks. Although there have been several studies of the effects of herbivory on C cycling (Sjögersten et al. 2010; Kelsey et al. 2016; Penner and Frank 2019; Koltz et al. 2022), the role of microbial communities in mediating these changes is not well known (Andriuzzi and Wall 2017). We suggest that herbivory studies that explicitly account for microbial community effects will improve our understanding of how herbivory influences climate change.
Conclusions
We show that the proportion of organic matter utilized increases with the rate of fresh C addition, and that the strength of this relationship is dependent upon the type of litter added. The nutrient content of litter only partially explains this phenomenon; shifts in microbial communities also play an important role. Moreover, our results demonstrate that animals have complex and far-reaching impacts on soil biogeochemistry through their impacts on litter chemistry and abundance, which to date, are not incorporated into predictive ecosystem models (Schmitz et al. 2018).
Data availability
Data are available at the NSF Arctic Data Center: https://doi.org/10.18739/A2CC0TV7W.
References
Andriuzzi WS, Wall DH (2017) Responses of belowground communities to large aboveground herbivores: meta-analysis reveals biome‐dependent patterns and critical research gaps. Glob Change Biol 23:3857–3868. https://doi.org/10.1111/gcb.13675
Beard KH, Choi RT, Leffler AJ et al (2019) Migratory goose arrival time plays a larger role in influencing forage quality than advancing springs in an Arctic coastal wetland. PLoS ONE 14:1–21. https://doi.org/10.1371/journal.pone.0213037
Beard KH, Kelsey KC, Choi RT et al (2023) Goose feces effects on subarctic soil nitrogen availability and greenhouse gas fluxes. Ecosystems 26:187–200. https://doi.org/10.1007/s10021-022-00752-x
Blagodatskaya E, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131. https://doi.org/10.1007/s00374-008-0334-y
Bolyen E, Rideout JR, Dillon MR et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
Callahan BJ, McMurdie PJ, Rosen MJ et al (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Camacho C, Coulouris G, Avagyan V et al (2009) BLAST+: architecture and applications. BMC Bioinform 10:421. https://doi.org/10.1186/1471-2105-10-421
Caporaso JG, Lauber CL, Walters WA et al (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA 108:4516–4522. https://doi.org/10.1073/pnas.1000080107
Caporaso JG, Lauber CL, Walters WA et al (2012) Ultra-high-throughput microbial community analysis on the illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. https://doi.org/10.1038/ismej.2012.8
Castellano MJ, Mueller KE, Olk DC et al (2015) Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob Change Biol 21:3200–3209. https://doi.org/10.1111/gcb.12982
Chemidlin Prévost-Bouré N, Soudani K, Damesin C et al (2010) Increase in aboveground fresh litter quantity over-stimulates soil respiration in a temperate deciduous forest. Appl Soil Ecol 46:26–34. https://doi.org/10.1016/j.apsoil.2010.06.004
Cleveland CC, Reed SC, Keller AB et al (2014) Litter quality versus soil microbial community controls over decomposition: a quantitative analysis. Oecologia 174:283–294. https://doi.org/10.1007/s00442-013-2758-9
Cotrufo MF, Wallenstein MD, Boot CM et al (2013) The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Biol 19:988–995. https://doi.org/10.1111/gcb.12113
Creamer CA, de Menezes AB, Krull ES et al (2015) Microbial community structure mediates response of soil C decomposition to litter addition and warming. Soil Biol Biochem 80:175–188. https://doi.org/10.1016/j.soilbio.2014.10.008
Crow SE, Lajtha K, Bowden RD et al (2009) Increased coniferous needle inputs accelerate decomposition of soil carbon in an old-growth forest. For Ecol Manag 258:2224–2232. https://doi.org/10.1016/j.foreco.2009.01.014
DeSantis TZ, Hugenholtz P, Larsen N et al (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. https://doi.org/10.1128/AEM.03006-05
Fanin N, Hättenschwiler S, Fromin N (2014) Litter fingerprint on microbial biomass, activity, and community structure in the underlying soil. Plant Soil 379:79–91. https://doi.org/10.1007/s11104-014-2051-7
Fernandes AD, Macklaim JM, Linn TG et al (2013) ANOVA-like differential expression (ALDEx) analysis for mixed population RNA-Seq. PLoS ONE 8:e67019. https://doi.org/10.1371/journal.pone.0067019
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364. https://doi.org/10.1890/05-1839
Foley KM, Beard KH, Atwood TB, Waring BG (2021) Herbivory changes soil microbial communities and greenhouse gas fluxes in a high-latitude wetland. Microb Ecol. https://doi.org/10.1007/s00248-021-01733-8
Fondell TF, Flint PL, Sedinger JS et al (2011) Intercolony variation in growth of black brant goslings on the Yukon–Kuskokwim Delta, Alaska. J Wildl Manag 75:101–108. https://doi.org/10.1002/jwmg.24
Frost CJ, Hunter MD (2008) Insect herbivores and their frass affect Quercus rubra leaf quality and initial stages of subsequent litter decomposition. Oikos 117:13–22. https://doi.org/10.1111/j.2007.0030-1299.16165.x
German DP, Chacon SS, Allison SD (2011) Substrate concentration and enzyme allocation can affect rates of microbial decomposition. Ecology 92:1471–1480. https://doi.org/10.1890/10-2028.1
German DP, Weintraub MN, Grandy AS et al (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43:1387–1397. https://doi.org/10.1016/j.soilbio.2011.03.017
Gessner MO (2005) Proximate lignin and cellulose. In: Graca MA, Barlocher F, Gessner MO (eds) Methods to study litter decomposition: a practical gude. Springer, Dordrecht, pp 115–120
Grandy AS, Neff JC (2008) Molecular C dynamics downstream: the biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci Total Environ 404:297–307. https://doi.org/10.1016/j.scitotenv.2007.11.013
Groemping U, Matthias L (2021) Package relaimpo: relative importance of regressors in linear models. R package version
Hicks LC, Yuan M, Brangarí A et al (2022) Increased above- and belowground plant input can both trigger microbial nitrogen mining in subarctic tundra soils. Ecosystems 25:105–121. https://doi.org/10.1007/s10021-021-00642-8
Ho A, Di Lonardo DP, Bodelier PLE (2017) Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol Ecol 93:1–14. https://doi.org/10.1093/femsec/fix006
Jorgenson MT (2000) Hierarchical organizations of ecosystems at multiple spatial scales on Yukon-Kuskokwim Delta, Alaska, U.S.A. Arctic. Antarct Alp Res 32:221–239. https://doi.org/10.1080/15230430.2000.12003360
Jorgenson T, Ely C (2001) Topography and flooding of coastal ecosystems on the Yukon–Kuskokwim delta, Alaska: implications for sea-level rise. J Coast Res 17:124–136
Kalbitz K, Kaiser K (2008) Contribution of dissolved organic matter to carbon storage in forest mineral soils. J Plant Nutr Soil Sci 171:52–60. https://doi.org/10.1002/jpln.200700043
Kalbitz K, Kaiser K, Bargholz J, Dardenne P (2006) Lignin degradation controls the production of dissolved organic matter in decomposing foliar litter. Eur J Soil Sci 57:504–516. https://doi.org/10.1111/j.1365-2389.2006.00797.x
Kelsey KC, Leffler AJ, Beard KH et al (2016) Interactions among vegetation, climate, and herbivory control greenhouse gas fluxes in a subarctic coastal wetland. J Geophys Res Biogeosci 121:2960–2975. https://doi.org/10.1002/2016JG003546
Klappenbach JA, Dunbar JM, Schmidt TM (2000) rRNA Operon copy number reflects ecological strategies of bacteria. Appl Environ Microbiol 66:1328–1333. https://doi.org/10.1128/AEM.66.4.1328-1333.2000
Klotzbücher T, Kaiser K, Guggenberger G, Kalbitz K (2011) A new model for the fate of lignin in decomposing. Ecology 95:1052–1062. https://doi.org/10.2307/41151233
Koltz AM, Gough L, McLaren JR (2022) Herbivores in Arctic ecosystems: effects of climate change and implications for carbon and nutrient cycling. Ann NY Acad Sci 1516:28–47. https://doi.org/10.1111/nyas.14863
Kuzyakov Y (2011) How to link soil C pools with CO2 fluxes? Biogeosciences 8:1523–1537. https://doi.org/10.5194/bg-8-1523-2011
Leffler AJ, Beard KH, Kelsey KC et al (2019) Delayed herbivory by migratory geese increases summer-long CO2 uptake in coastal western Alaska. Glob Change Biol 25:277–289. https://doi.org/10.1111/gcb.14473
Lenth R (2020) Emmeans: estimated marginal means, aka least-squares means. R Pack Vers 1(4):4
Li H, Yang S, Semenov MV et al (2021) Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob Change Biol 27:2763–2779. https://doi.org/10.1111/gcb.15593
Liang C, Amelung W, Lehmann J, Kästner M (2019) Quantitative assessment of microbial necromass contribution to soil organic matter. Glob Change Biol 25:3578–3590. https://doi.org/10.1111/gcb.14781
Liang C, Schimel JP, Jastrow JD (2017) The importance of anabolism in microbial control over soil carbon storage. Nat Microbiol. https://doi.org/10.1038/nmicrobiol.2017.105
Lin H, Peddada S (2020) Analysis of compositions of microbiomes with bias correction. Nat Commun 11:3514. https://doi.org/10.1038/s41467-020-17041-7
Liu X-JA, Sun J, Mau RL et al (2017) Labile carbon input determines the direction and magnitude of the priming effect. Appl Soil Ecol 109:7–13. https://doi.org/10.1016/j.apsoil.2016.10.002
Ma Y, Huang S, Gan Z et al (2020) The succession of bacterial and fungal communities during decomposition of two hygrophytes in a freshwater lake wetland. Ecosphere 11:e03242. https://doi.org/10.1002/ecs2.3242
Malik AA, Martiny JBH, Brodie EL et al (2020) Defining trait-based microbial strategies with consequences for soil carbon cycling under climate change. ISME J 14:1–9. https://doi.org/10.1038/s41396-019-0510-0
Mallick H, Rahnavard A, McIver LJ et al (2021) Multivariable association discovery in population-scale meta-omics studies. PLOS Comput Biol 17:e1009442. https://doi.org/10.1371/journal.pcbi.1009442
Manzoni S, Taylor P, Richter A et al (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196:79–91. https://doi.org/10.1111/j.1469-8137.2012.04225.x
Morrissey EM, Kane J, Tripathi BM et al (2023) Carbon acquisition ecological strategies to connect soil microbial biodiversity and carbon cycling. Soil Biol Biochem 177:108893. https://doi.org/10.1016/j.soilbio.2022.108893
Na M, Yuan M, Hicks LC, Rousk J (2022) Testing the environmental controls of microbial nitrogen-mining induced by semi-continuous labile carbon additions in the subarctic. Soil Biol Biochem 166:108562. https://doi.org/10.1016/j.soilbio.2022.108562
Nearing JT, Douglas GM, Hayes MG et al (2022) Microbiome differential abundance methods produce different results across 38 datasets. Nat Commun 13:342. https://doi.org/10.1038/s41467-022-28034-z
Nemergut DR, Knelman JE, Ferrenberg S et al (2016) Decreases in average bacterial community rRNA operon copy number during succession. ISME J 10:1147–1156. https://doi.org/10.1038/ismej.2015.191
Ngai JT, Jefferies RL (2004) Nutrient limitation of plant growth and forage quality in Arctic coastal marshes. J Ecol 92:1001–1010. https://doi.org/10.1111/j.0022-0477.2004.00926.x
Nilsson RH, Larsson K-H, Taylor AFS et al (2019) The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucl Acids Res 47:D259–D264. https://doi.org/10.1093/nar/gky1022
Oksanen J, Blanchet FG, Friendly M et al (2019) Vegan: community ecology package
Palecki M, Durre I, Applequist S et al (2021) U.S. Climate normals 2020: U.S. hourly climate normals (1991–2020) [Bethel, AK Weather Station]. In: NOAA Natl. Centers Environ. Inf
Penner JF, Frank DA (2019) Litter decomposition in yellowstone grasslands: the roles of large herbivores, litter quality, and climate. Ecosystems 22:929–937. https://doi.org/10.1007/s10021-018-0310-9
Person BT, Herzog MP, Ruess RW et al (2003) Feedback dynamics of grazing lawns: coupling vegetation change with animal growth. Oecologia 135:583–592. https://doi.org/10.1007/s00442-003-1197-4
Person BT, Ruess RW (2003) Stability of a subarctic saltmarsh: plant community resistance to tidal inundation. Écoscience 10:351–360. https://doi.org/10.1080/11956860.2003.11682784
Petkuviene J, Vaiciute D, Katarzyte M et al (2019) Feces from piscivorous and herbivorous birds stimulate differentially phytoplankton growth. Water 11:2567. https://doi.org/10.3390/w11122567
Pfister DH, Healy R (2021) Pezizomycetes. Encyclopedia of mycology. Elsevier, Amsterdam, pp 295–309
Pinheiro J, Bates D, Debroy S, Sarkar D (2019) nlme: Linear and nonlinear mixed effects models
R Core Team (2023) R: a language and enrironment for statistical computing
Roller BRK, Stoddard SF, Schmidt TM (2016) Exploiting rRNA operon copy number to investigate bacterial reproductive strategies. Nat Microbiol 1:1–7. https://doi.org/10.1038/nmicrobiol.2016.160
Schimel JP, Schaeffer SM (2012) Microbial control over carbon cycling in soil. Front Microbiol 3:1–11. https://doi.org/10.3389/fmicb.2012.00348
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563. https://doi.org/10.1016/S0038-0717(03)00015-4
Schmitz OJ, Wilmers CC, Leroux SJ et al (2018) Animals and the zoogeochemistry of the carbon cycle. Science. https://doi.org/10.1126/science.aar3213
Schnecker J, Bowles T, Hobbie EA et al (2019) Substrate quality and concentration control decomposition and microbial strategies in a model soil system. Biogeochemistry 144:47–59. https://doi.org/10.1007/s10533-019-00571-8
Sedinger JS, Chelgren ND, Ward DH, Lindberg MS (2008) Fidelity and breeding probability related to population density and individual quality in black brent geese Branta bernicla nigricans. J Anim Ecol 77:702–712. https://doi.org/10.1111/j.1365-2656.2008.01403.x
Shahbaz M, Kuzyakov Y, Heitkamp F (2017) Decrease of soil organic matter stabilization with increasing inputs: mechanisms and controls. Geoderma 304:76–82. https://doi.org/10.1016/j.geoderma.2016.05.019
Sjögersten S, van der Kuijper DPJ et al (2010) Nitrogen transfer between herbivores and their forage species. Polar Biol 33:1195–1203. https://doi.org/10.1007/s00300-010-0809-9
Stoddard SF, Smith BJ, Hein R et al (2015) rrnDB: improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucl Acids Res 43:D593–D598. https://doi.org/10.1093/nar/gku1201
Sun G, Zhu-Barker X, Chen D et al (2017) Responses of root exudation and nutrient cycling to grazing intensities and recovery practices in an alpine meadow: an implication for pasture management. Plant Soil 416:515–525. https://doi.org/10.1007/s11104-017-3236-7
Tande GF, Jennings TW (1986) Classification and mapping of tundra near Hazen Bay. Yukon Delta National Wildlife Refuge, Alaska
Thompson LR, Sanders JG, McDonald D et al (2017) A communal catalogue reveals earth’s multiscale microbial diversity. Nature 551:457–463. https://doi.org/10.1038/nature24621
Wardle DA, Bonner KI, Barker GM (2002) Linkages between plant litter decomposition, litter quality, and vegetation responses to herbivores. Funct Ecol 16:585–595. https://doi.org/10.1046/j.1365-2435.2002.00659.x
Witt C, Gaunt JL, Galicia CC et al (2000) A rapid chloroform-fumigation extraction method for measuring soil microbial biomass carbon and nitrogen in flooded rice soils. Biol Fertil Soils 30:510–519. https://doi.org/10.1007/s003740050030
Zhou H, He K, Chen J, Zhang X (2022) LinDA: linear models for differential abundance analysis of microbiome compositional data. Genome Biol 23:95. https://doi.org/10.1186/s13059-022-02655-5
Zhou W, Hui D, Shen W (2014) Effects of soil moisture on the temperature sensitivity of soil heterotrophic respiration: a laboratory incubation study. PLoS ONE 9:e92531. https://doi.org/10.1371/journal.pone.0092531
Acknowledgements
We thank R. Choi for lab assistance; Otter Kulmatiski helped with artwork on the graphical abstract; Battelle Polar Services for logistical assistance; Yukon Delta National Wildlife Refuge staff; and the people of Chevak, AK. This research was conducted under USFWS NWR Special Use Permit: 21-01.
Funding
This study was funded by National Science Foundation (NSF) (ARC-1932889) and an Early Career Research Fellowship to TBA from the Gulf Research Program of the National Academies of Sciences, Engineering, and Medicine (The content is solely the responsibility of the authors and does not necessarily represent the official views of the Gulf Research Program of the National Academies of Sciences, Engineering, and Medicine). This research was supported by the Utah Agricultural Experiment Station, Utah State University, and approved as journal paper number 9709.
Author information
Authors and Affiliations
Contributions
BGW, KHB, and TBA designed the experiments. TS, JA, and KHB performed the experiments. All authors analyzed the data. TS and JA wrote the original draft, KHB, BGW and TBA revised the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible editor: Jacques C Finlay.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saunders, T., Adkins, J., Beard, K.H. et al. Herbivores influence biogeochemical processes by altering litter quality and quantity in a subarctic wetland. Biogeochemistry 166, 67–85 (2023). https://doi.org/10.1007/s10533-023-01098-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-023-01098-9