Abstract
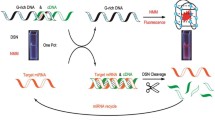
MicroRNA-21 (miRNA-21) is a kind of RNA that exists in biological fluids such as blood, urine and saliva. It has over expression in liver cancer and has different expression in different stages of cancer. However, due to the characteristics of small base number, short length, low abundance and easy degradation of miRNA-21, the detection of miRNA-21 is a challenging subject. Visualization, sensitive, specific and stable detection of tumor suppressor or oncogene microRNAs (miRNAs) remains challenging and is highly significant for clinical diagnostics. To solve this problem, we have developed a target-triggered hybridization assembly DNA machine for intracellular miRNA imaging based on strand displacement amplification (SDA) and branched hybridization chain reaction (B-HCR). In this approach, the target miRNA could hybridize with the template probe to trigger the SDA, resulting in the formation of nicked fragments (NFs) that hybridized with hairpin probe1 (HP1). The opened HP1 could hybridize with hairpin probe2 (HP2), leading to the self-assembly of hyperbranched DNA nanostructures through B-HCR. As expected, the newly developed method exhibits a detection limit down to 11.3 pM miRNA-21 and achieves high selectivity toward miRNA-21 against other interfering miRNAs. Due to its superior sensitivity and selectivity, our method can be further used to detect miRNA-21 in human serum samples. By taking advantage of intelligent design, the proposed method was also used for image miRNA-21 expression levels in different cell lines. This method shows a broad application in clinical diagnosis.
Graphical abstract







Similar content being viewed by others
References
V. Ambros, The functions of animal microRNAs. Nature 431(7006), 350 (2004)
H. Dong, J. Lei, L. Ding, Y. Wen, H. Ju, X. Zhang, MicroRNA: function, detection, and bioanalysis. Chem. Rev. 113(8), 6207–6233 (2013)
D.G. Spiller, C.D. Wood, D.A. Rand, M.R. White, Measurement of single-cell dynamics. Nature 465(7299), 736 (2010)
C.M. Croce, Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 10(10), 704–714 (2009)
W. Roa, B. Brunet, L. Guo, J. Amanie, A. Fairchild, Z. Gabos, T. Nijjar, R. Scrimger, D. Yee, J. Xing, Identification of a new microRNA expression profile as a potential cancer screening tool. Clin. Investig. Med. 33, 124–132 (2010)
Y. Tu, W. Li, P. Wu, H. Zhang, C. Cai, Fluorescence quenching of graphene oxide integrating with the site-specific cleavage of the endonuclease for sensitive and selective microRNA detection. Anal. Chem. 85(4), 2536–2542 (2013)
E. Várallyay, J. Burgyán, Z. Havelda, MicroRNA detection by northern blotting using locked nucleic acid probes. Nat. Protoc. 3(2), 190 (2008)
R. Deng, L. Tang, Q. Tian, Y. Wang, L. Lin, J. Li, Toehold-initiated rolling circle amplification for visualizing individual microRNAs in situ in single cells. Angew. Chem. Int. Ed. 53(9), 2389–2393 (2014)
R. Duan, X. Zuo, S. Wang, X. Quan, D. Chen, Z. Chen, L. Jiang, C. Fan, F. Xia, Lab in a tube: ultrasensitive detection of microRNAs at the single-cell level and in breast cancer patients using quadratic isothermal amplification. J. Am. Chem. Soc. 135(12), 4604–4607 (2013)
H. Dong, J. Lei, H. Ju, F. Zhi, H. Wang, W. Guo, Z. Zhu, F. Yan, Target-cell-specific delivery, imaging, and detection of intracellular microRNA with a multifunctional SnO2 nanoprobe. Angew. Chem. Int. Ed. 51(19), 4607–4612 (2012)
H. Peng, A.M. Newbigging, M.S. Reid et al., Signal amplification in living cells: a review of microRNA detection and imaging. Anal. Chem. 92(1), 292–308 (2020)
G. Yang, T. Song, M. Wang et al., Recent advancements in nanosystem-based molecular beacons for RNA detection and imaging. CS Appl. Nano Mater 3, 3065–3086 (2022)
L. Mo, D. Liang, M. Mo, C. Yang, W. Lin, Dual-detection of miRNAs in living cells via hybridization chain reaction onDNA tetrahedron. Sensors and Actuators: B. Chemical. 375(1), 132955–132963 (2023)
D. Zhu, J. Huang, B. Lu et al., Intracellular microRNA imaging with MoS2-supported nonenzymatic catassembly of DNA hairpins. ACS Appl. Mater. Interfaces 11(23), 20725–20733 (2019)
C. Li, J. Zhang, Y. Gao, S. Luo, Z.S. Wu, Nonenzymatic autonomous assembly of cross-linked network structures from only two palindromic DNA components for intracellular fluorescence imaging of miRNAs. ACS Sens. 7(2), 601–611 (2022)
H. Zhou, Y. Jiang, W. Zhao, S. Zhang, Light-activated nanodevice for on-demand imaging of miRNA in living cells via logic assembly. ACS Appl. Mater. Interfaces 14(11), 13070–13078 (2022)
H. Liu, C. Ma, S. Ge, M. Yan, J. Yu, Imaging of microRNA with enzyme-free signal amplification of catalyzed hairpin assembly in living cells. Nanomed. Nanotechnol. Biol. Med. 12(2), 512 (2016)
S.J. Zhen, X. Xiao, C.H. Li, C.Z. Huang, An enzyme-free DNA circuit-assisted graphene oxide enhanced fluorescence anisotropy assay for microRNA detection with improved sensitivity and selectivity. Anal. Chem. 89(17), 8766–8771 (2017)
T. Tian, H. Xiao, Z. Zhang, Y. Long, S. Peng, S. Wang, X. Zhou, S. Liu, X. Zhou, Sensitive and convenient detection of microRNAs based on cascade amplification by catalytic DNAzymes. Chem. A Eur. J. 19(1), 92–95 (2013)
Z. Zhou, Y.S. Sohn, R. Nechushtai, I. Willner, ACS Nano 14, 9021–9031 (2020)
F. Yang, Y. Cheng, Y. Zhang, W. Wei, H. Dong, H. Lu, X. Zhang, Anal. Chem. 92, 4411–4418 (2020)
J. Wang, Y. Gao, P. Liu, S. Xu, X. Luo, Anal. Chem. 92, 15169–15178 (2020)
J. Su, F. Wu, H. Xia, Y. Wu, S. Liu, Chem. Sci. 11, 80–86 (2020)
Acknowledgements
The authors gratefully acknowledge the financial support of the Medical and health science and technology project of Xiamen (3502Z20209179, 3502Z20194059).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no competing interest exists.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, J., Wang, M., Lin, P. et al. Trigger-activated autonomous DNA machine for amplified liver cancer biomarker microRNA21 imaging. ANAL. SCI. 39, 1661–1667 (2023). https://doi.org/10.1007/s44211-023-00397-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-023-00397-3