Abstract
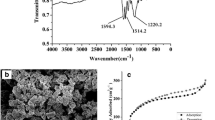
Azo-linked covalent organic polymers (ACOPs) were synthesized by a simple azo reaction, with 2,2ʹ-bis(trifluoromethyl)benzidine and 1,3,5-trihydroxybenzene as the monomers. The preparation process was mild, green, and environmental-friendly, avoiding the use of high temperature, metal catalysis, and harmful organic reagent. The obtained ACOPs were characterized by scanning electron microscopy, Fourier transform infrared spectroscopy, thermogravimetric analysis, powder X-ray diffraction, and Brunauer–Emmett–Teller. With the prepared ACOPs as adsorbent, a method of pipette tip solid-phase extraction–liquid chromatography–tandem mass spectrometry detection (PTSPE–LC–MS/MS) was proposed for the analysis of target sedatives in animal tissues. Furthermore, the parameters for the extraction of five sedatives, including the amount of adsorbent, pH value, ion strength, elution solvent and volume, were investigated. Under the optimized conditions, the linear dynamic range was found from 0.1 to 10.0 μg kg−1, and the limits of detection were ranged from 0.02 to 0.1 μg kg−1. The method was assessed by the analysis of target sedatives in animal tissues, and the recoveries for the spiked pork muscle and pork liver samples were 84–102% and 83–101%, respectively. The results show that the developed method of PTSPE–LC–MS/MS with ACOPs as adsorbent is efficient for the analysis of trace sedatives in animal tissues.
Graphical Abstract






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
N.P. Wu, F.G. Ban, L. Peng, H.X. Zhou, Z.T. Liu, H.W. Liu, J. Chin. Mass Spectrom. Soc. 33, 94–98 (2012)
L.L. Cheng, Y.J. Zhang, J.Z. Shen, C.M. Wu, S.X. Zhang, Chromatographia 71, 155–158 (2010)
L. Sun, L. Zhang, Q. Xu, S.H. Wang, X. Wang, Chin. J. Chromatogr. 28, 38–42 (2010)
L.J. Yan, J. Zhang, C.S. Pan, L.Y. Lin, X.Y. Zhang, H.Q. Shen, Chin. J. Anal. Chem. 41, 31–35 (2013)
L.P. Wang, H.X. Zhao, Y.M. Qiu, Z.Q. Zhou, J. Chromatogr. A 1136(1), 99–105 (2006)
G. Zhang, A.V. Jr Terry, M.G. Bartlett, J. Chromatogr. B 854(1–2), 68–76 (2007)
L.Q. Zhang, P.G. Wu, Q. Jin, Y.M. Zhang, X.F. Wang, R. Ren, Chin. J. Health Lab. Technol. 23, 1634–1648 (2013)
GB 31650–2019 National food safety standard maximum residue limits of veterinary drugs in food.
S.A. Meenagh, J.D.G. McEvoy, C.T. Elliott, Anal. Chim. Acta 462, 149–156 (2002)
J. Cooper, P. Delahaut, T.L. Fodey, C.T. Elliott, Analyst 129, 169–174 (2004)
V. Cerkvenik-Flajs, Anal. Chim. Acta 586, 374–382 (2007)
L. Zhang, P. Wu, Q. Jin, H. Ye, X. Huang, S. Liu, Food Anal. Methods 10, 354–362 (2017)
Y. Aoki, H. Hakamata, Y. Igarashi, K. Uchida, H. Kobayashi, N. Hirayama, A. Kotani, F. Kusu, J. Chromatogr. B 877, 166–172 (2009)
L. Zhang, P. Wu, Q. Jin, Z. Hu, J. Wang, J. Chromatogr. B 1072, 305–314 (2018)
J. Zhang, B. Shao, J. Yin, Y. Wu, H. Duan, J. Chromatogr. B 877, 1915–1922 (2009)
K. Mitrowska, A. Posyniak, J. Zmudzki, Anal. Chim. Acta 637, 185–192 (2009)
Y. Ning, Y. Ye, W. Liao, Y. Xu, W. Wang, A. Wang, Food Chem. 397, 133831 (2022)
H. Sun, J. Feng, J. Feng, M. Sun, Y. Feng, M. Sun, Food Chem. 395, 133633 (2022)
R. Sun, F. Lu, C. Yu, Y. Yang, L. Qiao, A. Liu, J. Chromatogr. A 1673, 463101 (2022)
J. Yu, S. Di, H. Yu, T. Ning, H. Yang, S. Zhu, J. Chromatogr. A 1637, 461822 (2021)
Y. Yuan, S. Liang, H. Yan, Z. Ma, Y. Liu, J. Chromatogr. A 1408, 49–55 (2015)
T. Ben, Y. Li, L. Zhu, D. Zhang, D. Cao, Z. Xiang, X. Yao, S. Qiu, Energy Environ. Sci. 5(8), 8370–8376 (2012)
S. Lin, C.S. Diercks, Y.B. Zhang, N. Kornienko, E.M. Nichols, Y. Zhao, A.R. Paris, D. Kim, P. Yang, O.M. Yaghi, C.J. Chang, Science 349(6253), 1208–1213 (2015)
G. Ji, Z. Yang, H. Zhang, Y. Zhao, B. Yu, Z. Ma, Z. Liu, Angew. Chem. Int. Ed. 55(33), 9685–9689 (2016)
G. Ji, Z. Yang, H. Zhang, Y. Zhao, B. Yu, Z. Ma, Z. Liu, Angew. Chem. 128, 9837–9841 (2016)
D. Li, M. He, B. Chen, B. Hu, J. Chromatogr. A 1601, 1–8 (2019)
Y. Yamini, M. Faraji, J. Pharm. Anal. 4(4), 279–285 (2014)
S. Song, X. Shi, R. Li, Z. Lin, A. Wu, D. Zhang, Process. Biochem. 43, 1209–1214 (2008)
G. Zhang, J.A.V. Terry, M.G. Bartlett, J. Chromatogr. B 854, 68–76 (2007)
H.R. Sobhi, Y. Yamini, R. Haji Hosseini Baghdad Abadi, J. Pharm. Biomed. Anal. 45, 769–774 (2007)
C. Li, W.G. Huang Pu, Y. Ting, Y.L. Wu, Chin. J. Anal. Chem. 7, 1015–1018 (2010)
Funding
This work was supported by the fund from Training Program for Academic and Technical Leaders of Major Disciplines in Jiangxi Province, China (20213BCJ22008), Basic research and Talent training Program of Jiangxi Academy of Agricultural Sciences (JXSNKYJCRC 202201), and the National quality and safety risk assessment of livestock and poultry products in 2022 (GJFP20220301).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
On behalf of all the author, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiang, J.J., Yuan, L.J., Liao, Q.G. et al. Synthesis of azo-linked covalent organic polymers for pipette tip solid-phase extraction of sedative residues from animal tissues samples. ANAL. SCI. 39, 1939–1946 (2023). https://doi.org/10.1007/s44211-023-00406-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-023-00406-5