Abstract
Background
Patent ductus arteriosus (PDA) in preterm infants is associated with increased morbidities and mortality. Prophylactic treatment with cyclooxygenase inhibitors, as indomethacin or ibuprofen, failed to demonstrate significant clinical benefits. Acetaminophen may represent an alternative treatment option.
Objective
This study evaluated the minimum effective dose of prophylactic acetaminophen to close the ductus and assessed the safety and tolerability profile in extremely preterm infants at 23–26 weeks of gestation.
Methods
A dose finding trial with Bayesian continual reassessment method was performed in a multicenter study with premature infants hospitalized in neonatal intensive care unit. Infants of 23–26 weeks of gestation and post-natal age ≤ 12 h were enrolled. Four intravenous acetaminophen dose levels were predefined. The primary outcome was the ductus arteriosus closing at two consecutive echocardiographies or at day 7. The main secondary objectives included the safety of acetaminophen on hemodynamics and biological hepatic function.
Results
A total of 29 patients were analyzed sequentially for the primary analysis with 20 infants assigned to the first dose level followed by 9 infants to the second dose level. No further dose level increase was necessary. The posterior probabilities of success, estimated from the Bayesian logistic model, were 46.1% [95% probability interval (PI), 24.9–63.9] and 67.6% (95% PI, 51.5–77.9) for dose level 1 and 2, respectively. A closing or closed pattern was observed among 19 patients at the end of treatment [65.5% (95% confidence interval (CI), 45.7–82.0)]. No change in alanine aminotransferase values was observed during treatment. A significant decrease in aspartate aminotransferase values was observed with postnatal age. No change in systolic and diastolic blood pressures was observed during treatment.
Conclusions
Minimum effective dose to close the ductus was 25 mg/kg loading dose then 10 mg/kg/6 h for 5 days in extremely preterm infants. Acetaminophen was well tolerated in this study following these doses.
Trial Registration
ClinicalTrials.gov Identifier: NCT04459117.
Similar content being viewed by others
Previous studies have shown that prophylactic treatment of patent ductus arteriosus with cyclooxygenase inhibitors does not demonstrate significant clinical benefits |
Acetaminophen may represent an alternative treatment option. This study evaluated the minimum effective dose and assessed the safety and tolerability profile of acetaminophen in extremely preterm infants at 23–26 weeks of gestation. |
Acetaminophen was well tolerated and the minimum effective dose was 25 mg/kg loading dose then 10 mg/kg/6hours during 5 days |
1 Introduction
At birth, the ductus arteriosus is functionally open in all newborns and its constriction and closure are part of the normal process of postnatal adaptation. In extremely preterm infants, failure to close is frequent and results in a condition called patent ductus arteriosus (PDA), which has been associated with increased mortality and morbidity [1].
Inhibition of prostaglandin synthesis, through inhibition of the cyclooxygenase (COX) enzymes, results in ductal constriction. Indomethacin and ibuprofen are the two most commonly used COX inhibitors in presence of a hemodynamically significant PDA. However, numerous adverse effects have been reported in this context, such as bleeding, immune disorders, renal impairment, gastrointestinal hemorrhage, necrotizing enterocolitis, intestinal perforation, and, for some cases, pulmonary hypertension [2, 3]. Treatments aiming at closing the PDA have failed to demonstrate significant clinical benefits [4] and current evidence does not support the use of prophylactic indomethacin [5] or ibuprofen [6] for the prevention of PDA-related morbidities.
Early treatment of PDA with acetaminophen has been proposed as an alternative to COX inhibitors. According to a recent meta-analysis, use of acetaminophen has a comparable effectiveness to close a PDA, and fewer harmful effects on the kidneys and intestines [5]. However, only few extremely preterm infants were included in this meta-analysis. Therefore, efficacy and safety of acetaminophen for PDA treatment in this population required further studies.
Both animal and clinical studies demonstrated that the responsiveness of the PDA to acetaminophen could depend on the method of administration, duration of treatment, and dose of the drug. Ductal response to acetaminophen has been highly variable, ranging from no efficacy in infants receiving a short course of oral acetaminophen to closure in most of the patients who received the intravenous form, suggesting that a critical acetaminophen level is required to achieve maximal therapeutic effect [6]. Data from a randomized double-blind controlled trial performed in Finland [7] showed that acetaminophen accelerates early closure of the PDA. A modeling analysis of the data showed that gestational age was strongly associated with time to ductus closure and that maximal effect of acetaminophen was associated also with gestational age [8].
In the Prophylactic Treatment of the Ductus Arteriosus in Preterm Infants by Acetaminophen project (TREOCAPA) (NCT04459117), we planned the present phase II study for the high-risk preterm infant population born at 23–26 weeks of gestation. The aim was to define the minimum effective dose and assess the safety profile of acetaminophen in this population. A continual reassessment methodology was chosen because of the advantages of this design especially in such vulnerable population.
2 Methods
2.1 Study Design and Participants
This phase 2 trial enrolled patients from eight sites across two countries (France and Finland). Patients were assigned to intravenous acetaminophen with predefined doses. The first level was 20 mg/kg followed by 7.5 mg/kg quarter in die (QID) during 5 days (total = 20 doses) [7, 8]. The second, third, and fourth predefined level doses stand for 25 mg/kg followed by 10 mg/kg QID, 30 mg/kg followed by 12 mg/kg QID, and 35 mg/kg followed by 15 mg/kg QID, respectively.
Infants were eligible for inclusion if the following conditions were all met: (1) birth at 23–26 weeks gestation, (2) postnatal age < 12 h, and (3) authorization to perform the study signed by both parents after receiving an information letter written by European representative parents. Noninclusion criteria were the presence of a birth defect or congenital anomaly, twin-to-twin transfusion syndrome, suspicion of pulmonary hypoplasia or hepatic failure (hemorrhagic syndrome and/or severe hypoglycemia), clinical instability that can lead to rapid death, and the participation in another clinical trial using acetaminophen during the first 5 days of life or indomethacin or ibuprofen during the first 3 days of life.
2.2 Procedures
At inclusion, baseline data (pregnancy, antenatal care, and care in the delivery room) were recorded. Systemic arterial pressure was measured before the loading dose of acetaminophen. Systemic arterial pressure was measured after each dose. A blood sample was collected just after end of loading dose infusion to measure aspartate aminotransferase (AST), alanine aminotransferase (ALT) levels, and for pharmacokinetic purpose. After the tenth dose, a blood sample was collected to analyze AST and ALT levels. Echocardiography was performed each day during the first postnatal week (day 1 to day 7). The first echocardiography was performed at day 1 after initiation of treatment. A cerebral echography was also performed at day 7. A last visit was planned at 36 weeks of postmenstrual age for reviewing severe morbidity, including bronchopulmonary dysplasia, brain lesions, infections, retinopathy of prematurity, and necrotizing enterocolitis. The first three consecutive patients were treated at the lowest starting dose level. Thereafter, a Bayesian continual reassessment analysis was used to assign the dose level after each three consecutive patients [9]. The Data Safety Monitoring Committee (DSMC) was consulted before any increase of dose level.
2.3 Outcomes
The main objective of phase II was to evaluate the minimum effective dose of acetaminophen (MEDR) to close PDA in infants of 23–26 weeks gestation. PDA closure was defined as PDA closed at two consecutive echocardiographies or at echocardiography performed on day 7. Patients who were switched from acetaminophen to ibuprofen for rescue treatment were considered as treatment failures. The secondary objectives were the tolerability and safety of acetaminophen with the following outcomes: blood pressure assessed 30, 60, 90, 120 min after end of each drug infusion and ALT and AST levels assessed after administration of the first dose and the tenth dose. Serious adverse events during the first 7 days of life and during follow-up until the last visit at 36 weeks of postmenstrual age were also assessed. Post hoc analysis included assessment of efficacy criteria as percentage of patients with closing or closed ductal pattern at the end of treatment (i.e., day 5).
2.4 Statistical Analysis
A sample size of 30 preterm infants with gestational age of 23–26 weeks were planned to be enrolled. Statistical analysis regarding efficacy endpoints was performed on the per-protocol (PP) set. This PP included patients without major protocol violations regarding eligibility criteria and with the primary endpoint recovered. This dose-finding study was conducted using a Bayesian continual reassessment method (see Supplementary Material text for model parametrization). The PDA closure rate used to define the MEDR was fixed to 60%, which is above the spontaneous PDA closure rate and close to the success rate reported with prophylactic use of indomethacin in preterm infants < 26 weeks [10]. The reported rate of spontaneous PDA closure at day 7 in preterm infants with gestational age of 23–26 weeks ranges from 13 to 32% [11]. Categorical data were summarized and presented as frequencies and percentages. Continuous data were presented as median [interquartile range, IQR] (range). Comparisons of quantitative data were performed using Wilcoxon–Mann–Whitney tests and categorical variables were analyzed using the chi-square test or Fisher’s exact test as appropriate. Paired Wilcoxon tests were used to analyze changes from baseline value. Statistical analysis was performed on R software (http://cran.r-project.org/). All tests were two-sided with a significance level of 5%.
3 Results
3.1 Efficacy Analysis
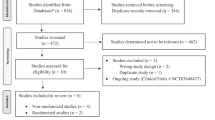
Between November 2020, and September 2021, a total of 31 preterm patients were enrolled in this study. One patient was excluded from analysis due to noninclusion criteria and one patient stopped the treatment earlier for suspicion of safety concern and was not retained in the primary efficacy analysis. A total of 29 patients were sequentially analyzed, 20 in the first dose level and 9 in the second dose level (Fig. 1, Table 1). After inclusion of 20 successive patients in the first dose level (20 mg/kg loading dose then 7.5 mg/kg/6 h for 5 days), 9 cumulative failures related to the primary outcome were observed (11 successes). Among the 9 failures, 3 patients needed to receive a rescue treatment by ibuprofen during the acetaminophen time course and for 6 patients, none of the echocardiographies performed from day 1 to 7 showed a closed PDA. A dose escalation to the second level was decided after the seventh Bayesian reassessment analysis and was approved by the DSMC. Nine additional consecutive patients were treated thereafter with 25 mg/kg loading dose then 10 mg/kg/6 h for 5 days. Among these patients, six were considered as a failure regarding the primary outcome (three successes). The failures are detailed thereafter: one patient was switched from acetaminophen to ibuprofen as a rescue treatment during the treatment course, two patients received a rescue treatment by ibuprofen after day 5, and, for three patients, none of the echocardiographies performed throughout the follow-up showed a PDA closed. Given the predefined acceptable success probability, the data suggested that no gain on efficacy would be observed with further increase in dose level. Based on overall data, the posterior efficacy probabilities (95% probability interval) estimated from the Bayesian logistic model were 46.1% (24.9–63.9%) and 67.6% (51.5–77.9%) for dose level 1 and 2 respectively. Therefore, dose level 2 was chosen as the MEDR. Figure 2 shows the sequential estimated probability of success along with its 95% credibility intervals associated with the MEDR of acetaminophen after each analysis. The model-predicted probabilities of success as a function of dose levels are also provided in Supplementary Fig. 1. Comparison of baseline characteristics of patients between success and failure patients regarding the primary endpoint are provided in Supplementary Table 1.
The post hoc analysis showed that a total of 19 preterm infants over the 29 analyzed patients, i.e., 65.5% (95% CI, 45.7–82.0), displayed a closing ductal pattern or a ductus closed at day 5 corresponding to end of treatment. Distribution of PDA shunt flow pattern (%) per day of acetaminophen treatment is displayed in Supplementary Fig. 2. Stratification on the dose level showed that 13 patients (65%) in dose level one displayed a closing ductal pattern, or a ductus closed, at day 5 versus 6 patients (67%) in dose level two. The mean percent change of PDA diameter during treatment from the first echocardiography is shown also in Supplementary Fig. 3. The percent change stratified by dose level for left atrium/aorta ratio, left ventricular end-diastolic diameter, left pulmonary artery mean velocity, and left pulmonary artery end-diastolic velocity is provided in Supplementary Fig. 4. Supplementary Fig. 5 displays the time course of ductus diameter/birthweight, left atrium/aorta ratio, left ventricular end-diastolic diameter, and left pulmonary artery end-diastolic velocity from start to end of acetaminophen treatment, stratified by failure or success regarding the primary endpoint.
To assess acetaminophen metabolism rate in these extremely preterm patients, concentrations of acetaminophen and its metabolites (acetaminophen–sulfate, acetaminophen–glucuronide, and acetaminophen–cysteine/mercapturate) were measured just after end of the loading dose infusion. Supplementary Fig. 6 shows acetaminophen and metabolites concentrations according to success or failure regarding the primary endpoint. No difference was observed for the parent drug concentrations at end of loading dose suggesting no difference in acetaminophen volume of distribution between success and failure patients. It is noteworthy that for a given drug, volume of distribution mainly drives loading dose peak concentration. However, higher inactive metabolite levels were observed in failure patients particularly for acetaminophen glucuronide and acetaminophen–cysteine/mercapturate (p < 0.05 and p = 0.07, respectively) even after adjusting for gestational age.
3.2 Safety Analysis
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured after the first dose and after dose number 10. No difference was observed in ALT levels between dose number 1 and number 10 with either dose level 1 or dose level 2 (Fig. 3). However, a significant decrease in AST values (whatever the dose level) was shown suggesting a normalization of the AST values occurring with postnatal age.
Baseline median (IQR) systolic and diastolic blood pressure before intravenous (IV) acetaminophen were 51 (44–57) and 29 (26–33) mmHg, respectively. After the first dose, the median (IQR) systolic blood pressures 30, 60, 90, 120 min after end of drug infusion were 47 (44–59), 48 (42–57), 45 (42–57), and 47 (42–58) mmHg respectively. The median (IQR) diastolic blood pressures were 28 (22–38), 31 (23–38), 27 (22–36), 32 (25–36) respectively. No statistical difference was pointed out for both systolic and diastolic blood pressures compared with baseline (P > 0.05). Systolic and diastolic blood pressure time course is shown in Supplementary Fig. 7.
Among patients who received at least one dose of study medication, a total of 27 serious adverse events (SAE) occurred in 13 (41.9%) patients. The most frequent SAE, gastrointestinal disorders, occurred in nine patients (29%). Among the patients in failure group, two patients had a gastrointestinal event during the first week (two necrotizing enterocolitis, respectively, at day 5 and at day 7) and three patients had a gastrointestinal event beyond the first week of life (one necrotizing enterocolitis at day 9 and two intestinal perforations, respectively, at day 9 and day 19). Four preterm infants (12.9%) died during the study period—three born at 24 weeks of gestation and one at 23 weeks of gestation. None of deaths were considered to be related to the study drug or procedures. Table 2 summarizes all AE and SAE reported during the follow-up of this study and stratifies it by dose level.
4 Discussion
This is the first study that assess by Bayesian approach the minimum effective dose of acetaminophen for patent ductus arteriosus in extremely preterm infants at 23–26 weeks gestation. This minimum effective dose was estimated to be 25 mg/kg followed by 10 mg/kg QID for 5 days. This is in agreement with a previous study that suggested that 10 mg/kg/6 h should be enough to treat PDA with no need to increase further the dose in preterm infants at 26 weeks gestation [12].
The continual reassessment methodology was preferred over a parallel group design because of the multiple advantages of this design especially in such vulnerable population. This sequential dose increases the approach to avoid unnecessary overexposure of these at-risk patients. This approach allows also the enrollment of fewer patients to determine the minimum effective dose response without requirement of a placebo group. Furthermore, a suboptimal range of acetaminophen doses can be pointed out earlier. Additionally, rather than considering each dose level as independent conditions, this approach allows for enriched data observation using prior beliefs and accounts for pharmacological assumption that each dose is interdependent. Due to the small sample size, considering that each dose level is not interconnected would lead to a range for the second dose level with a large uncertainty regarding the primary endpoint rate—33% with a wide 95% confidence interval, from 7.5 to 70.1%. The continual reassessment design allows for incorporation of overall cumulated successes in the trial, assuming a monotonic increase of the probability of success as dose level increases. This Bayesian inference allows for a posteriori estimate of success rate probability, along with its credibility/probability interval (PI) incorporating contextual information (see Supplementary Material text) with more informative estimations. The 95% PI estimation of success rate for the second dose level was narrower from 51.5 to 77.9%. A slight misbalance in gestational age, birth weight, and use of mechanical ventilation was observed between the two dose levels. However, none of these variables were significantly associated with treatment success, suggesting that no confounding effects of these variables should be considered in this extreme preterm cohort.
The rates of PDA in preterm infants at day 7 after birth was reported to be 68% in infants with gestational ages 25–26 weeks and rising to 87 % for 24-week-old infants [11]. Regarding these previous reported findings, the use of intravenous acetaminophen appears to decrease the PDA rate in these extremely premature infants. Interestingly, our results also show that prophylactic acetaminophen use allowed for patients to be considered as a “failure” (with the study primary criterion), with values close to the success patients for left pulmonary artery end-diastolic velocity and left atrium/aorta ratio. Additionally, the mean left atrium/aorta ratio was below 1.5 during the treatment time course for both success and failure patients (value considered as a cutoff to discriminate hemodynamically significant PDA) [13]. These observations strengthen the benefits of this prophylactic dose regimen on overall patients.
A marked variability of plasma acetaminophen and concentrations of its metabolites was observed between subjects after the loading dose. This is in agreement with a previous pharmacokinetic studies performed in a preterm population that reported high interindividual variabilities on both acetaminophen elimination clearance and volume of distribution [8, 14]. Plasma inactive metabolite levels measured after end-of-loading dose were higher in preterm infants in whom ductus closure was not observed, which can support also the use of the higher dosage proposed in dose level 2 overdose level 1. However, further specific pharmacokinetic studies are needed to better characterize the distribution and elimination of acetaminophen in these extremely preterm infants along with the association between drug exposure and the ductus diameter time course.
Hepatic toxicity of acetaminophen has been reported when plasma concentrations exceeded largely the therapeutic levels. This complication is attributed to the production of highly reactive metabolites by hepatic cytochrome P (CYP)-450, primarily by the CYP2E1 [15]. In our study, no significant variation in ALT levels was pointed out during acetaminophen treatment in dose level 1 nor in dose level 2. Regarding AST values, we observed a significant decrease as a function of time during treatment towards normal values with postnatal age. In this cohort of patients, no hepatic alteration was suspected during or after the 5 days of treatment.
The hemodynamic effect of acetaminophen was previously studied and only a very modest decrease in heart rate and mean arterial blood pressure was reported on prospectively collected observations in 72 neonates with no clinical relevance [16]. This agrees with our findings, as no significant alteration in hemodynamic parameters were observed based on systolic and diastolic blood pressure measured before and 30, 60, 90, 120 min after end of each drug infusion.
A total of 27 SAE occurred in 13 patients. The most affected organ was the gastrointestinal system, including eight patients with either necrotizing enterocolitis or gastrointestinal perforation. In a recent study, general incidence of spontaneous intestinal perforation was estimated around 8% in infants < 29 weeks gestational age. In our study, we observed specifically gastrointestinal perforation at rate of 13% (95% CI, 3.6–29%). Regarding the association between GI SAEs and treatment failure, no statistical difference was pointed out regarding the gastrointestinal SAE rate among patients in failure or not (33% versus 14%, respectively p = 0.39). However, the low sample size may limit interpretation of these results.
Among the eight patients with either necrotizing enterocolitis or gastrointestinal perforation, three were assessed as possibly related to the study drug by investigators. Interestingly, all eight patients received hydrocortisone that might have increased the risk of spontaneous gastrointestinal perforation [17]. A suspicion of safety concern was raised, and these cases were reviewed with members of the DSMC. However, the DSMC estimated that extremely premature infants are a high-risk population with many expected complications and that the pattern of events we observed was consistent with what might be observed in these infants. Interestingly, most of the patients presenting a gastrointestinal SAE received prophylactic treatment by hydrocortisone, known to have gastrointestinal adverse events. In most of the cases (seven out of eight cases of GI event), acetaminophen administration had been completed or stopped several days before the occurrence of GI events, while hydrocortisone treatment was still ongoing in most of the cases (six cases out of eight cases of GI event). Thus, and given the known pharmacologic properties and half-life of acetaminophen in neonates (around 4 h) [8], chronology seems more compatible with an effect of hydrocortisone. Moreover, the proportion of patients treated by hydrocortisone was unbalanced between dose level 1 and 2 (91% versus 56%), which might explain the lower observed gastrointestinal disorder incidence rate in dose level 2. Besides, preterm infants with slightly higher birth weight and lesser use of mechanical ventilation in dose level 2 might explain also in part the lower SAE rate. Gastrointestinal complications were considered as special-interest events and will be carefully monitored in phase III. In the same way, the safety in the long term is yet to be proven. The more patients exposed to acetaminophen, especially those at lower risk, the higher the chance it will alter the benefit. This will be considered and investigated in phase III. The benefit–risk balance was the basis of this dose escalation phase II study with the aim to avoid unnecessary overexposure to acetaminophen in this vulnerable population.
The survival rate in the study was relatively high given the gestational age included in this study. A total of four deaths (12.9%; 95% CI 3.6–29.8%) were observed, all corresponding to birth < 25 weeks gestation. Interestingly, the mortality rate observed in this study appears substantially lower than those reported in two previous French studies, the PREMILOC interventional study [18] and the EPIPAGE observational study [24].
In conclusion, according to the present phase II study, the minimum effective dose included 25 mg/kg loading dose, followed by 10 mg/kg/6 h for 5 days for extremely preterm infants. Acetaminophen was well tolerated in this study. Phase III, the second part of the TREOCAPA project, is ongoing to evaluate the interest of acetaminophen using a clinically relevant endpoint such as survival without severe morbidity compared with placebo.
References
Hamrick SEG, Sallmon H, Rose AT, et al. Patent ductus arteriosus of the preterm infant. Pediatrics. 2020;146: e20201209.
Sivanandan S, Agarwal R. Pharmacological closure of patent ductus arteriosus: selecting the agent and route of administration. Paediatr Drugs. 2016;18:123–38.
Gournay V, Savagner C, Thiriez G, et al. Pulmonary hypertension after ibuprofen prophylaxis in very preterm infants. Lancet Lond Engl. 2002;359:1486–8.
Benitz WE, Committee on Fetus and Newborn, American Academy of Pediatrics. Patent ductus arteriosus in preterm infants. Pediatrics. 2016. https://doi.org/10.1542/peds.2015-3730.
Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010;2010:CD000174.
Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev. 2018;9:CD003481.
Härkin P, Härmä A, Aikio O, et al. Paracetamol accelerates closure of the ductus arteriosus after premature birth: a randomized trial. J Pediatr. 2016;177:72-77.e2.
Bouazza N, Treluyer J-M, Foissac F, et al. pharmacokinetics of intravenous paracetamol (acetaminophen) and ductus arteriosus closure after premature birth. Clin Pharmacol Ther. 2021;110:1087–95.
O’Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics. 1990;46:33–48.
Narayanan M, Cooper B, Weiss H, et al. Prophylactic indomethacin: factors determining permanent ductus arteriosus closure. J Pediatr. 2000;136:330–7.
Su B-H, Lin H-Y, Chiu H-Y, et al. Therapeutic strategy of patent ductus arteriosus in extremely preterm infants. Pediatr Neonatol. 2020;61:133–41.
Balasubramanian H, Jain V, Bhalgat P, et al. Low dose paracetamol for management of patent ductus arteriosus in very preterm infants: a randomised non-inferiority trial. Arch Dis Child Fetal Neonatal Ed. 2022. https://doi.org/10.1136/archdischild-2022-323781.
Iyer P, Evans N. Re-evaluation of the left atrial to aortic root ratio as a marker of patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 1994;70:F112–7.
Cook SF, Roberts JK, Samiee-Zafarghandy S, et al. Population pharmacokinetics of intravenous paracetamol (acetaminophen) in preterm and term neonates: model development and external evaluation. Clin Pharmacokinet. 2016;55:107–19.
Lee SS, Buters JT, Pineau T, et al. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–7.
Allegaert K, Naulaers G. Haemodynamics of intravenous paracetamol in neonates. Eur J Clin Pharmacol. 2010;66:855–8.
Shaffer ML, Baud O, Lacaze-Masmonteil T, et al. Effect of prophylaxis for early adrenal insufficiency using low-dose hydrocortisone in very preterm infants: an individual patient data meta-analysis. J Pediatr. 2019;207:136-142.e5.
Baud O, Maury L, Lebail F, et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet Lond Engl. 2016;387:1827–36.
Cheung YK. Dose finding by the continual reassessment method. CRC Press; 2011.
Lee SM, Cheung YK. Model calibration in the continual reassessment method. Clin Trials Lond Engl. 2009;6:227–38.
Yuan Y, Nguyen HQ, Thall PF. Bayesian designs for phase I-II clinical trials. CRC Press; 2017.
Acknowledgments
The authors thank the members of the Data Safety Monitoring Committee: Patrick Mc Namara, University of Iowa, USA; Roger Soll, University of Vermont; USA; Keith Barrington, University of Montreal, Canada; Christine Durier, Paris-Est University, France; Florentia Kaguelidou, Université Paris Cité, France. The authors thank the members of the steering committee: Corinne Alberti, University of Paris-Diderot, France; Moreno Ursino, University of Paris-Diderot, France; Pierre Yves Ancel, University of Paris-Descartes, France; Jayanta Banerjee, Imperial College Healthcare NHS Trust, London, UK; Olivier Baud, University of Geneva, Switzerland; Ricardo Carbajal, University of Paris-Descartes, France; Olivier Claris, Hospices Civils de Lyon, France; Eugene Demsey, Cork University, Ireland; Anne Greenough, King’s College Hospital NHS Foundation Trust, London, UK; Gorm Greisen, Copenhagen University Hospital, Denmark; Pierre-Henri Jarreau, University of Paris-Descartes, France; Pierre Kuhn, University of Strasbourg, France; Nail Marlow, University College London, UK; Stéphane Marret, University of Rouen, France; Christoph Rûegger, University of Zürich, Switzerland; Jennifer Zeitlin, University of Paris-Descartes, France; Laurent Flet, Nantes University hospital, France. The authors thank Marina Dumousseaux and Aure Vanhecke from Inserm Clinical research department and Naouel Bouafia for implementation of the study. The authors thank the Pediatric Clinical Research Network in France (Pedstart) team. The authors thank Magali Herve for data management of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Naïm Bouazza, Gilles Cambonie, Cyril Flamant, Aline Rideau, Manon Tauzin, Juliana Patkai, Géraldine Gascoin, Mirka Lumia, Outi Aikio, Gabrielle Lui, Léo Froelicher Bournaud, Aisling Walsh-Papageorgiou, Marine Tortigue, Alban-Elouen Baruteau, Jaana Kallio, Mikko Hallman, Alpha Diallo, Léa Levoyer, Jean-Marc Treluyer And Jean-Christophe Roze declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
The trial was approved by ethics committee of Centre Hospitalier La Chartreuse under the number approval SI 20.03.09.40128 for France and by the regional medical research ethics committee of North Ostrobothnia under the number approval 68/06.00.00/20 19 for Finland.
Data Availability Statement
The data that support the findings of this study are available from Institut national de la santé et de la recherche médicale (Inserm), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Inserm.
Authors' Contributions
NB, AD, LL and JC had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: NB, MH, JMT, JCR. Acquisition, analysis, or interpretation of data: NB, AD, LL and JCR Drafting of the manuscript: NB, LL, JCR. Critical revision of the manuscript for important intellectual content: all authors.
Funding
Institut national de la santé et de la recherche médicale (Inserm) is the sponsor of the Treocapa trial. The project leading to this application has received funding through the Connect4Children consortium from the Innovative Medicines Initiatives 2 Join Undertaking under Grant Agreement no. 777389. This Joint Undertaking receives the support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
Role of the Funder/sponsor
The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The publication reflects the author's view and neither Innovative Medicines Initiatives (IMI) nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained therein.
Consent to Participate
Written informed consent was obtained from both parents.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bouazza, N., Cambonie, G., Flamant, C. et al. Prophylactic Intravenous Acetaminophen in Extremely Premature Infants: Minimum Effective Dose Research by Bayesian Approach. Pediatr Drugs 26, 83–93 (2024). https://doi.org/10.1007/s40272-023-00602-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00602-w