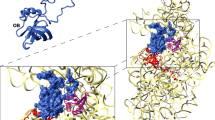
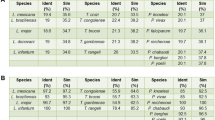
Abstract
Initiation of translation is the first of the three obligatory steps required for protein synthesis and is carried out by a large number of protein factors called initiation factors in conjunction with ribosomes. One of the key conserved protein factors in eukaryotes that plays a role in this process is eIF4A, which has three homologues in humans with eIF4A1 being the primary factor playing a role in translation initiation. eIF4As are members of the family of DEAD-box helicases that carry out different biological functions. eIF4A1s are recruited to translation initiation complexes via association with eIF4G and have ATP binding, ATP hydrolysis, RNA binding, and unwinding activities. Plasmodium and trypanosomatids such as Leishmania and Trypanosoma are parasites that cause human disease. While mechanistically the function of eIF4A1s in eukaryotes is well-understood, the orthologues peIF4A1s and keIF4A1s in Plasmodium and trypanosomatids are not well-studied. Here, we have used bioinformatics tools and homology modelling/structure prediction to study the motifs and functional signatures of Plasmodium and trypanosomatid peIF4A1s/keIF4A1s. We report a high degree of sequence conservation, structural conservation, and conservation of protein–protein interaction signatures of Plasmodium and trypanosomatid peIF4A1s/keIF4A1s in comparison with human eIF4A1. Thus, in spite of the great divergence in evolution between these parasites and higher eukaryotes, there is remarkable conservation of motifs and functional signatures in Plasmodium and trypanosomatid peIF4A1s/keIF4A1s.






Similar content being viewed by others
References
Andreou AZ and Klostermeier D 2013 The DEAD-box helicase eIF4A: paradigm or the odd one out? RNA Biol. 10 19–32
Blum S, Schmid SR, Pause A, et al. 1992 ATP hydrolysis by initiation factor 4A is required for translation initiation in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 89 7664–7668
Conroy SC, Dever TE, Owens CL, et al. 1990 Characterization of the 46,000-dalton subunit of eIF-4F. Arch. Biochem. Biophys. 282 363–371
Das S 2021 Taking a re-look at cap-binding signatures of the mRNA cap-binding protein eIF4E orthologues in trypanosomatids. Mol. Cell. Biochem. 476 1037–1049
Das S 2022 Analysis of domain organization and functional signatures of trypanosomatid keIF4Gs. Mol. Cell. Biochem. 477 2415–2431
Dhalia R, Reis CR, Freire ER, et al. 2005 Translation initiation in Leishmania major: characterisation of multiple eIF4F subunit homologues. Mol. Biochem. Parasitol. 140 23–41
Dhalia R, Marinsek N, Reis CR, et al. 2006 The two eIF4A helicases in Trypanosoma brucei are functionally distinct. Nucleic Acids Res. 34 2495–2507
Eswar N, Webb B, Marti-Renom MA, et al. 2006 Comparative protein structure modeling using Modeller. Curr. Protoc. Bioinformatics 5 5–6
Feoktistova K, Tuvshintogs E, Do A, et al. 2013 Human eIF4E promotes mRNA restructuring by stimulating eIF4A helicase activity. Proc. Natl. Acad. Sci. USA 110 13339–13344
Freire ER, Dhalia R, Moura DM, et al. 2011 The four trypanosomatid eIF4E homologues fall into two separate groups, with distinct features in primary sequence and biological properties. Mol. Biochem. Parasitol. 176 25–36
Freire ER, Vashisht AA, Malvezzi AM, et al. 2014 eIF4F-like complexes formed by cap-binding homolog TbEIF4E5 with TbEIF4G1 or TbEIF4G2 are implicated in post-transcriptional regulation in Trypanosoma brucei. RNA 20 1272–1286
Freire ER, Sturm NR, Campbell DA, et al. 2017 The role of cytoplasmic mRNA cap-binding protein complexes in Trypanosoma brucei and other trypanosomatids. Pathogens 6 55
Galicia-Vazquez G, Cencic R, Robert F, et al. 2012 A cellular response linking eIF4AI activity to eIF4AII transcription. RNA 18 1373–1384
Gingras AC, Raught B and Sonenberg N 2001 Regulation of translation initiation by FRAP/mTOR. Genes Dev. 15 807–826
Ho CK and Shuman S 2001 A yeast-like mRNA capping apparatus in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 98 3050–3055
Ignatochkina AV, Takagi Y, Liu Y, et al. 2015 The messenger RNA decapping and recapping pathway in Trypanosoma. Proc. Natl. Acad. Sci. USA 112 6967–6972
Jackson RJ, Hellen CU and Pestova TV 2010 The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11 113–127
Jumper J, Evans R, Pritzel A, et al. 2021 Highly accurate protein structure prediction with AlphaFold. Nature 596 583–589
Liang L, Diehl-Jones W and Lasko P 1994 Localization of vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities. Development 120 1201–1211
Lu WT, Wilczynska A, Smith E, et al. 2014 The diverse roles of the eIF4A family: you are the company you keep. Biochem. Soc. Trans. 42 166–172
Marintchev A 2013 Roles of helicases in translation initiation: a mechanistic view. Biochim. Biophys. Acta 1829 799–809
Marintchev A, Edmonds KA, Marintcheva B, et al. 2009 Topology and regulation of the human eIF4A/4G/4H helicase complex in translation initiation. Cell 136 447–460
Meijer HA, Kong YW, Lu WT, et al. 2013 Translational repression and eIF4A2 activity are critical for microRNA-mediated gene regulation. Science 340 82–85
Moura DM, Reis CR, Xavier CC, et al. 2015 Two related trypanosomatid eIF4G homologues have functional differences compatible with distinct roles during translation initiation. RNA Biol. 12 305–319
Oberer M, Marintchev A and Wagner G 2005 Structural basis for the enhancement of eIF4A helicase activity by eIF4G. Genes Dev. 19 2212–2223
Pause A and Sonenberg N 1992 Mutational analysis of a DEAD box RNA helicase: the mammalian translation initiation factor eIF-4A. EMBO J. 11 2643–2654
Pause A, Methot N and Sonenberg N 1993 The HRIGRXXR region of the DEAD box RNA helicase eukaryotic translation initiation factor 4A is required for RNA binding and ATP hydrolysis. Mol. Cell. Biol. 13 6789–6798
Pettersen EF, Goddard TD, Huang CC, et al. 2004 UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25 1605–1612
Pradhan A and Tuteja R 2007 Bipolar, dual Plasmodium falciparum helicase 45 expressed in the intraerythrocytic developmental cycle is required for parasite growth. J. Mol. Biol. 373 268–281
Rogers GW, Richter NJ and Merrick WC 1999 Biochemical and kinetic characterization of the RNA helicase activity of eukaryotic initiation factor 4A. J. Biol. Chem. 274 12236–12244
Rozen F, Pelletier J, Trachsel H, et al. 1989 A lysine substitution in the ATP-binding site of eucaryotic initiation factor 4A abrogates nucleotide-binding activity. Mol. Cell. Biol. 9 4061–4063
Schmid SR and Linder P 1991 Translation initiation factor 4A from Saccharomyces cerevisiae: analysis of residues conserved in the D-E-A-D family of RNA helicases. Mol. Cell. Biol. 11 3463–3471
Schutz P, Bumann M, Oberholzer AE, et al. 2008 Crystal structure of the yeast eIF4A-eIF4G complex: an RNA-helicase controlled by protein–protein interactions. Proc. Natl. Acad. Sci. USA 105 9564–9569
Sengoku T, Nureki O, Nakamura A, et al. 2006 Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell 125 287–300
Sokabe M and Fraser CS 2017 A helicase-independent activity of eIF4A in promoting mRNA recruitment to the human ribosome. Proc. Natl. Acad. Sci. USA 114 6304–6309
Stuart K, Brun R, Croft S, et al. 2008 Kinetoplastids: related protozoan pathogens, different diseases. J. Clin. Invest. 118 1301–1310
Tanner NK, Cordin O, Banroques J, et al. 2003 The Q motif: a newly identified motif in DEAD box helicases may regulate ATP binding and hydrolysis. Mol. Cell 11 127–138
Tauber D, Tauber G, Khong A, et al. 2020 Modulation of RNA condensation by the DEAD-Box protein eIF4A. Cell 180 411–426
Tuteja R and Pradhan A 2009 Isolation and functional characterization of eIF4F components and poly(A)-binding protein from Plasmodium falciparum. Parasitol. Int. 58 481–485
Tuteja R and Pradhan A 2010 PfeIF4E and PfeIF4A colocalize and their double-stranded RNA inhibits Plasmodium falciparum proliferation. Commun. Integr. Biol. 3 611–613
Tuteja R, Malhotra P, Song P, et al. 2002 Isolation and characterization of an eIF-4A homologue from Plasmodium cynomolgi. Mol. Biochem. Parasitol. 124 79–83
Tuteja R, Tuteja N, Malhotra P, et al. 2003 Replication fork-stimulated eIF-4A from Plasmodium cynomolgi unwinds DNA in the 3’ to 5’ direction and is inhibited by DNA-interacting compounds. Arch. Biochem. Biophys. 414 108–114
Williams-Hill DM, Duncan RF, Nielsen PJ, et al. 1997 Differential expression of the murine eukaryotic translation initiation factor isogenes eIF4A(I) and eIF4A(II) is dependent upon cellular growth status. Arch. Biochem. Biophys. 338 111–120
Yoder-Hill J, Pause A, Sonenberg N, et al. 1993 The p46 subunit of eukaryotic initiation factor (eIF)-4F exchanges with eIF-4A. J. Biol. Chem. 268 5566–5573
Acknowledgements
We thank Dr Shubbir Ahmed, All India Institute of Medical Sciences, New Delhi, India, for helpful discussions.
Funding
This work was not funded.
Author information
Authors and Affiliations
Contributions
Conceptualization, data curation, formal analysis, methodology, writing, and reviewing of this manuscript was done by SD. Homology modelling and co-writing of this manuscript was done by AD.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there are no competing interests.
Additional information
Corresponding editor: Sudha Bhattacharya
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dadwal, A., Das, S. Architecture, domain organization, and functional signatures of trypanosomatid keIF4A1s and Plasmodium peIF4A1s suggest conserved functions. J Biosci 48, 44 (2023). https://doi.org/10.1007/s12038-023-00369-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-023-00369-9