Abstract
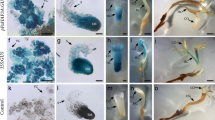
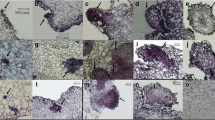
Somatic embryogenesis (SE) is a key regeneration process in plant. AcSERK1 is a gene specifically expressed in the early stage of SE in pineapple (Ananas comosus), suggesting that the promoter of SERK1 might contain specific cis-acting element regulating SE. To identify embryonic cell-specific element in the SERK1 promoter, a series of binary plant transformation vectors with GUS (β-glucuronidase) reporter gene were systematically analyzed by transient gene expression system in wild-type and transgenic pineapple embryogenic callus. Histochemical and quantitative GUS assays demonstrated that the activity of the AcSERK1 upstream regulatory sequence lacking − 921 to -911 or -910 to -880 was significantly reduced in the embryonic callus of the pineapple, and these two regions were needed for the embryonic cell-specific. Besides, a promoter lacking − 943 to -922 was shown to significantly increase GUS activity in embryogenic callus, suggesting repressive elements exist in this region. Our data of stable transformation assays confirmed again the 5’ upstream regulatory sequence (-921 to -880) of the AcSERK1 gene is an essential functional region. Our findings lay the basis for better understanding of the molecular mechanisms of AcSERK1 gene in the regulation in early stage of SE.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Allen RD, Bernier F, Lessard PA, Beachy RN (1989) Nuclear factors interact with a soybean -conglycinin enhancer. Plant Cell 1:623–631
Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208
Bhuria M, Goel P, Kumar S, Singh AK (2016) The promoter of AtUSP is cco-regulated by phytohormones and abiotic stresses in arabidopsis thaliana. Front Plant Sci 7:1957
Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu CM, van Lammeren AA, Miki BL, Custers JB, van Lookeren Campagne MM (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14:1737–1749
Braybrook SA, Harada JJ (2008) LECs go crazy in embryo development. Trends Plant Sci 13:624–630
Calheiros MBP, Vieira LGE, Fuentes SRL (1994) Effects of exogenous polyamines on direct somatic embryogenesis in coffee. Bras Fisiol Veg 6:109–114
Ciolkowski I, Wanke D, Birkenbihl RP, Somssich IE (2008) Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Mol Biol 68:81–92
de Silva KK, Dunwell JM, Wickramasuriya AM (2022) Weighted gene correlation Network Analysis (WGCNA) of Arabidopsis somatic embryogenesis (SE) and identification of key gene modules to uncover SE-associated hub genes
Elhiti M, Stasolla C (2013) Wang A.M. Molecular regulation of plant somatic embryogenesis. In Vitro Cell Dev Biol Plant 49:631–642
Elmayan T, Tepfer M (1995) Evaluation in tobacco of the organ specificity and strength of the rolD promoter, domain a of the 35S promoter and the 35S2 promoter. Transgenic Res 4:388–396
Fatihi A, Boulard C, Bouyer D, Baud S, Dubreucq B, Lepiniec L (2016) Deciphering and modifying LAFL transcriptional regulatory network in seed for improving yield and quality of storage compounds. Plant Sci 250:198–204
Gaj MD, Zhang S, Harada JJ, Lemaux PG (2005) Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta 222:977–988
Gao Y, Zan XL, Wu XF, Yao L, Chen YL, Jia SW, Zhao KJ (2014) Identification of fungus-responsive cis-acting element in the promoter of Brassica juncea chitinase gene, BjCHI1. Plant Sci 215:190–198
Grosveld F, van Staalduinen J, Stadhouders R (2021) Transcriptional regulation by (Super) Enhancers: from Discovery to Mechanisms. Annu Rev Genom Hum Genet 22
He YH, Luo J, Wu HT, Wang RX, Gao AP, Zhao CX, Yu XL, Ye ZX, Wang ZH, Han JZ, Liu HP (2007) Somatic embryogenesis from leaf base callus of Ananas comosus. Fruit Sci 24:59–63
Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt ED, Boutilier K, Grossniklaus U, de Vries SC (2001) The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant physiol 127:803–816
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27:297–300
Horstman A, Li MF, Heidmann I, Weemen MK, Chen BJ, Muino JM, Angenent GC, Boutilier K (2017) The BABY BOOM transcription factor activates the LEC1-ABI3-FUS3-LEC2 network to induce somatic embryogenesis. Plant Physiol 175:848–857
Ikeda M, Mitsuda N, Ohme-Takagi M (2009) Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell 21:3493–3505
Ikeda M, Takahashi M, Fujiwara S, Mitsuda N, Ohme-Takagi M (2020) Improving the efficiency of adventitious shoot induction and somatic embryogenesis via modification of WUSCHEL and LEAFY COTYLEDON 1. Plants 9:1434
Ikeuchi M, Sugimoto K, Iwase A (2013) Plant callus: mechanisms of induction and repression. Plant Cell 25:3159–3173
Jiménez-Guillen D, Pérez-Pascual D, Souza-Perera R, Godoy-Hernández G, Zúñiga-Aguilar JJ (2018) Cloning of the Coffea canephora SERK1 promoter and its molecular analysis during the cell-to-embryo transition. Electron J Biotechnol 36:34–46
Kithmee KDS, Jim MD, Anushka MW (2022) Weighted Gene Correlation Network Analysis (WGCNA) of Arabidopsis Somatic Embryogenesis (SE) and Identification of Key Gene Modules to Uncover SE-Associated Hub Genes”, International Journal of Genomics, vol. 2022, Article ID 7471063, 24 pages
Lescot M, De’hais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouz P, Rombauts S (2002) Plant CARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327
Li MF, Wrobel-Marek J?Heidmann I, Horstman A, Chen BJ, Reis R, Angenent GC, Boutilier K (2022) Auxin biosynthesis maintains embryo identity and growth during BABY BOOM-induced somatic embryogenesis. Plant Physiol 188: 1095–1110
Liu ZZ, Wang JL, Huang X, Xu WH, Liu ZM, Fang RX (2003) The promoter of a rice glycine-rich protein gene, Osgrp-2, confers vascular-specific expression in transgenic plants. Planta 216:824–833
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25:402–408
Lotan T, Ohto M, Yee KM, West MAL, Lo R, Kwong RW, Yamagishi K, Fischer RL, Goldberg RB, Harada JJ (1998) Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93:1195–1205
Loyolavargas V, Delapeña C, Galazávalos R, Quirozfigueroa F (2008) Plant tissue culture. In: Walker J, Rapley R (eds) Molecular Biomethods Handbook, 2nd edn. Humana Press, New York, NY, USA, pp 875–904
Luan AP, He YH, Lin WQ, Chen CJ, Feng JT, Xie T, Gong X, Xia JX (2016) Identification of transcription start site of AcSERK1 and its embryo specific cell promoter in pineapple. J Hortic 43:2251–2256
Luan AP, He YH, Xie T, Chen CJ, Mao Q, Wang XS, Li CH, Ding YQ, Lin WQ, Liu C, Xia JX, He JH (2019) Identification of an embryonic cell-specific region within the pineapple SERK1 promoter. Genes 10:883
Luan AP, Chen CJ, Xie T, He JH, He YH (2020) Methylation analysis of CpG islands in pineapple SERK1 promoter. Genes 2020, 11: 425–435
Ma J, He Y, Wu C, Liu H, Hu Z, Sun G (2012) Cloning and molecular characterization of a SERK gene transcriptionally induced during somatic embryogenesis in Ananas comosus cv. Shenwan. Plant Mol Biol Rep 30:195–203
Ma J, He YH, Hu ZY, Xu WT, Xia JX, Guo CH, Lin SQ, Chen CJ, Wu CH, Zhang JL (2014) Characterization of the third SERK gene in pineapple (Ananas comosus) and analysis of its expression and autophosphorylation activity in vitro. Genet Mol biology 37:530–539
Manisha V, Bansal YK (2004) Somatic embryogenesis and plantlet regeneration in semul (Bombax ceiba). Plant Cell Tiss Organ Cult 79:115–118
Mariashibu TS, Subramanyam K, Arun M, Mayavan S, Rajesh M, Theboral J, Manickavasagam M, Ganapathi A (2013) Vacuum infiltration enhances the Agrobacterium-mediated genetic transformation in indian soybean cultivars. Acta Physiol Plant 35:41–54
Mathew MW, Philip VJ (2003) Somatic embryogenesis versus zygotic embryogenesis in Ensete superbum. Plant Cell Tiss Organ Cult 72:267–275
Mozgová I, Muñoz-Viana R, Hennig L (2017) PRC2 represses Hormone-Induced somatic embryogenesis in vegetative tissue of Arabidopsis thaliana. PLoS Genet 13:e1006562
Nic-Can GI, Pena C (2014) Epigenetic advances on somatic embryogenesis of agronomical and important crops. In: Alvarez-Venegas R (ed) Epigenetics in plants of agronomic importance: Fundamentals and Applications, 6th edn. Springer International Publishing, Berlin, Germany, pp 91–109
Nolan KE, Kurdyukov S, Rose RJ (2009) Expression of the SOMATIC EMBRYOGENESIS RE-CEPTOR-LIKE KINASE1 (SERK1) gene is associated with developmental change in the life cycle of the model legume Medicago truncatula. J Exp Bot 60:1759–1771
Pandey DK, Chaudhary B (2014) Oxidative stress responsive SERK1 gene directs the progression of somatic embryogenesis in cotton (Gossypium hirsutum L. cv. Coker 310). Am J Plant Sci 2014:80–102
Paul P, Joshi S, Tian R, Junior RD, Manohar Chakrabarti M, Perry SE (2022) The MADS-domain factor AGAMOUS-Like18 promotes somatic embryogenesis. Plant Physiol 188:1617–1631
Pérez-Pascual D, Jiménez‐Guillen D, Villanueva‐Alonzo H, Souza‐Perera R, Godoy‐Hernández G, Zúñiga‐Aguilar JJ (2018) Ectopic expression of the Coffea canephora SERK1 homolog‐induced differential transcription of genes involved in auxin metabolism and in the developmental control of embryogenesis. Physiol Plant 163:530–551
Reinert J (1958) Under the control of the morphogenes and the induction of adventive embryos into tissues of litres of carrots. Plant 53:318–333
Rocha DI, Pinto DL, Vieira LM, Tanaka FA, Dornelas MC, Otoni WC (2016) Cellular and molecular changes associated with competence acquisition during passion fruit somatic embryogenesis: ultrastructural characterization and analysis of SERK gene expression. Protoplasma 253:595–609
Sakhanokho H, Rajasekaran K (2016) Cotton regeneration in vitro. In: Ramawat KG, Ahuja MR (eds) Fiber plants. Springer, Berlin, Germany, pp 87–110
Santos MO, Aragão FJL (2009) Role of SERK genes in plant environmental response. Plant Signal Behav 4:1111–1113
Schmidt E, Guzzo F, Toonen MA, De Vries SC (1997) A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124:2049–2062
Serfling E, Jasin M, Schaffner W (1985) Enhancers and eukaryotic gene transcription. Trends Genet 1:224–230
Shirsat A, Wilford N, Croy R, Boulter D (1989) Sequences responsible for the tissue specific promoter activity of a pea legumin gene in tobacco. Mol Gen Genet MGG 215:326–331
Singh A, Khurana P (2017) Ectopic expression of Triticum aestivum SERK genes (TaSERKs) control plant growth and development in Arabidopsis. Sci Rep 7:12368
Steiner N, Santa-Catarina C, Guerra MP, Cutri L, Dornelas MC, Floh EI (2012) A gymnosperm homolog of SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE-1 (SERK1) is expressed during somatic embryogenesis. Plant Cell Tiss Organ Cult 109:41–50
Steward FC, Mapes MO, Smith J (1958) Growth and organized development of cultured cells I. Growth and division of freely suspended cells. Am J Bot 45:693–703
Talukder P, Mitra D (2020) Expression of somatic embryogenesis receptor kinase (SERK) gene and its regulation under the influence of exogenous additives during in vitro somantic embryo development in medicinal plants. Am J Appl Biotechnol Res 1:1–10
Vogel G (2005) How does a single somatic cell become a whole plant? Science 309:86
Wang H, Han J, Kanagarajan S, Lundgren A, Brodelius PE (2013) Trichome-specific expression of the amorpha-4,11-diene 12-hydroxylase (cyp71av1) gene, encoding a key enzyme of artemisinin biosynthesis in Artemisia annua, as reported by a promoter-gus fusion. Plant Mol Biol 81:119–138
Xiao Y, Chen Y, Ding Y, Wu J, Wang P, Yu Y, Ge X (2018) Effects of GhWUS from upland cotton (Gossypium hirsutum L.) on somatic embryogenesis and shoot regeneration. Plant Sci 270:157–165
Xie T, Chen CJ, Li CH, Liu JR, Liu CY, He YH (2018) Genome-wide investigation of WRKY gene family in pineapple: evolution and expression profiles during development and stress. BMC Genomics 19:490
Yadak JS, Rajam MV (1997) Spatial distribution of free and conjugated polyamines in leaves of Solanum melongena L. associated with differential morphogenetic capacity: efficient somatic embryogenesis with putrescine. J Exp Bot 48:1537–1545
Yang F, Xia XR, Ke X, Ye J, Zhu LH (2020a) Somatic embryogenesis in slash pine (Pinus elliottii Engelm): improving initiation of embryogenic tissues and maturation of somatic embryos. Plant Cell Tiss Organ Cult 143:159–171
Yang Y, Wang N, Zhao S (2020b) Functional characterization of a WRKY family gene involved in somatic embryogenesis in Panax ginseng. Protoplasma 257:449–458
Zakizadeh H, Stummann BM, Lutken H, Muller R (2010) Isolation and characterization of four somatic embryogenesis receptor-like kinase (RhSERK) genes from miniature potted rose (Rosa hybrida cv. Linda). Plant Cell Tissue Organ cult 101:331–338
Zeng F, Zhang X, Cheng L, Hu L, Zhu L, Cao J, Guo X (2007) A draft gene regulatory network for cellular totipotency reprogramming during plant somatic embryogenesis. Genomics 90:620–628
Zhang ZL, Xie Z, Zou X, Casaretto J, Ho TH, Shen QJ (2004) A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol 134:1500–1513
Zhao DM, Zheng YX, Yang LG, Yao ZY, Cheng JF, Zhang F, Jiang HY, Liu D (2021) The transcription factor AtGLK1 acts upstream of MYBL2 to genetically regulate sucrose-induced anthocyanin biosynthesis in Arabidopsis. BMC Plant Biol 21:1–14
Zuo J, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30:349–359
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. 32101584), Natural Science Foundation of Guangdong Province (2020A1515110331), and Rural Revitalization Strategy Special Fund Seed Industry Revitalization Project of Guangdong Province (2022-NPY-00-031). The authors acknowledge support from National Tropical Plants Germplasm Resource Center.
Author information
Authors and Affiliations
Contributions
Y.-H.H. and T.X. conceived the research plan and designed the study. T.X. performed most of the experiments and data analysis. A.-P.L., C.-J.C., X.-S.W. and C.-Y.L. carried out part of material preparation. T.X. and Y.-H.H. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest between the authors.
Additional information
Communicated by Cecile Segonzac.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, T., Zhang, W., Chen, C. et al. Identification of a novel promoter region responsible for the embryo-specific expression of SERK1 in pineapple. Hortic. Environ. Biotechnol. 64, 1071–1082 (2023). https://doi.org/10.1007/s13580-023-00542-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-023-00542-x