Abstract
Lepidium ostleri S.L. Welsh & Goodrich (Ostler’s peppergrass) is an endemic plant species restricted to Ordovician limestone outcrops associated with the San Francisco Mountain Range in western Utah. Due to restricted population distribution and proximity to modern mining operations, L. ostleri is a species of conservation interest. This study focused on the development of a micropropagation protocol for propagating mature plants using plant tissue culture methods. Indirect shoot organogenesis was obtained from L. ostleri explants on Murashige and Skoog (MS) medium augmented with various concentrations of BAP (6-Benzylaminopurine), kinetin (N6-furfuryladenine), and IAA (indole-3-acetic acid). Plantlets supporting shoots grown in vitro were pulse treated with differing strengths of indole-3-butyric acid (IBA) and transferred to sterile soil. Following root induction, plantlets were acclimated to ambient conditions. The successful development of a micropropagation protocol supports management activities for L. ostleri and also contributes to in vitro propagation knowledge at the species, genus, and family levels.
Similar content being viewed by others
Introduction
Lepidium ostleri S.L. Welsh & Goodrich (Ostler’s peppergrass) is an edaphic endemic species narrowly restricted to Ordovician limestone outcrops associated with the San Francisco Mountain Range in western Utah. All known populations occur within boundaries of private mining patents where its habitat has sustained periods of concentrated mining activity since the late 1800s (Evenden 1998; USFWS 2011). Due to restricted population distribution and potential impact from large-scale modern mining operations, L. ostleri is a species of conservation interest; it is a Bureau of Land Management (BLM) sensitive plant species and was previously designated as a candidate for federal threatened status listing by The U.S. Fish and Wildlife Service (USFWS 2011).
In vitro methods have been explored to expand conservation tools for other rare plants in Utah, such as the endangered endemic species Ranunculus aestivalis (Pence et al. 2008) and Astragalus holmgreniorum (Hill et al. 2015). In vitro propagation techniques offer a powerful suite of conservation tools for threatened and rare plants (Sarasan et al. 2006; Paunescu 2009; Engelmann 2011; Cruz-Cruz et al. 2013). While traditional propagation methods may often provide sufficient and accessible means of obtaining plants for conservation activities, such as population augmentation or introduction, plant tissue culture approaches may be beneficial for species where source tissue for explants or seed availability is limited (Bunn et al. 2010). Rare plants with relatively small distributions or limited access may be good candidates for alternative propagation methods.
Micropropagation protocols typically comprise three general stages. The first stage often involves the production of adventitious shoots from embryogenic callus or directly from wounded explant tissue via de novo shoot organogenesis in response to various growth regulators. While methods for inducing organogenesis or embryogenesis have not been published for L. ostleri, somatic embryogenesis and shoot organogenesis have been described for other Lepidium species (Osuna et al. 2006; Polzerovà et al. 2011). Multiple shoot proliferation from L. sativum shoot tip explants has been reported in response to various concentrations of kinetin and BAP (Sandhya et al. 2016), as well as IAA and BAP (Bhasin et al. 2015).
The intermediate stage typically involves root induction in shoots using in vitro or ex vitro methods. Ex vitro rooting has several advantages over in vitro rooting; plantlets developed from ex vitro rooting are reported to have more developed root systems, higher survival rates, and greater success when acclimating. Additionally, micropropagation protocols utilizing ex vitro rooting may require less labor and time for completion (Phulwaria et al. 2013). Common procedures for ex vitro rooting are shared across many plant families; Passiflora edulis and Passiflora foetida (Passifloraceae), Bauhinia racemosa (Fabaceae), and Ceropegia bulbosa (Apocynaceae) have all reported successful responses to pulse treatment, or the submersion of the plantlet base in phytohormone solution, for 3 to 7 min using 100 to 400 mg L−1 IBA before transfer to soil (Phulwaria et al. 2013; Shekhawat et al. 2015a, 2015b; Sharma et al. 2017). Similarly, Leptadenia reticulata (Apocynaceae) developed roots when pulse treated with a 125:125 mM solution of IBA and β-naphthoxyacetic acid (NOA) (Arya et al. 2003). Pulse-treated plantlets should be given time for root growth to initiate before moving to the final stage of micropropagation (Arya et al. 2003; Phulwaria et al. 2013; Shekhawat et al. 2015a).
The final stage typically involves the acclimatization of rooted plantlets to the external environment through continually increasing the exposure time of plants to ambient conditions. Acclimatization of L. virginicum and L. sativum Linn. has been successful through the transfer of rooted plants to covered containers of soil, wet either with water, partial strength MS salts, or partial strength MS liquid media, and a period of 2 to 4 wk of humidity reduction and MS salt/media concentration reduction if applicable before transfer to the greenhouse or field (Pande et al. 2002; Osuna et al. 2006; Bhasin et al. 2015). Plantlets that are successfully acclimatized increase in photosynthetic capabilities and develop the ability to regulate transpiration rates (Pospisilova et al. 1999; Hazarika et al. 2006).
The purpose of this study was to develop a micropropagation protocol for L. ostleri that was composed of three major experimental phases. The first phase was to produce adventitious shoots from explants via direct or indirect organogenesis in response to various ratios of N6-furfuryladenine (kinetin) and indole-3-acetic acid (IAA) to 6-Benzylaminopurine (BAP) using leaf explants. The second phase was to induce ex vitro rooting of plantlets supporting shoots using pulse treatments of various concentrations of indole-3-butyric acid (IBA) and transfer to soil. The last phase was to acclimatize rooted plantlets to the external environment by continually increasing the exposure time of plants to ambient conditions before their transfer to the greenhouse.
Materials and methods
Tissue source and preparation
Tissue was collected from in situ populations for precursory exploratory experiments. Due to the seasonal unavailability of field tissue during full-scale experiments, explant tissue for the BAP:IAA experiments was obtained from plants grown from seed in greenhouse conditions and cultured directly onto PGR treatment media. Terminal stem segments with multiple young, healthy leaves that did not show apparent physical signs of flower or fruit development were selected. Shoot clusters were cleaned of debris and leaves displaying signs of dieback or disease were discarded. Leaves were washed in a 50.0 mL tap water solution containing 2 drops Triton X-100 (Sigma, St. Louis, MO) for 10 min and rinsed 3 times with tap water. They were then surface sterilized in a 0.5% sodium hypochlorite (NaOCl) solution with 0.5 mL of Triton X-100 for 10 min, rinsed 5 times with sterile deionized water, and transferred to a sterile dish. For the BAP:Kinetin experiments, an 8-wk-old in vitro-grown plant was selected for explant excisions. This plant was grown on Murashige and Skoog (MS; Murashige and Skoog 1962) culture medium augmented with 0.1 mg L−1 IAA and 2.0 mgL−1 BAP and subsequently transferred to media without PGRs.
Shoot proliferation
A three-level full factorial design was used to test both BAP:Kinetin and BAP:IAA concentration ratios (Table 1). Approximately 3- to 5-mm leaf tip and petiole explants were aseptically excised from sterile tissue of mature plants and individually inoculated in sterile culture vessels containing MS basal medium with 1.0% agar, an adjusted pH of 5.7, and PGRs by treatment (Table 1). Explants were incubated at 25 ± 2 °C with a 16-h photoperiod.
Due to the development of dense vegetative growth, treatments were analyzed by examining plant size rather than shoot number. This was accomplished by photographing explants displaying shoot response weekly, allowing for the capture and analysis of plant growth; this analytical method is being used with increasing frequency to quantify growth and other parameters (Spalding and Miller 2013; Clark et al. 2020; Li et al. 2020). Two photos per plantlet were taken each week from two different aspects (Aspect 1, and Aspect 2 rotated 90° from Aspect 1). Photos were shot in a lightbox with two mounted diffused white lights using a Canon Coolpix B500 camera (300 dpi, f/5.1, 1/400 s, ISO-125, 26 mm focal length, 3.2 max aperture). Photos were analyzed using ImageJ 1.x (Schneider et al. 2012) image processing software (Fig. 1). Images were converted to an 8-bit graphic, and the image threshold was adjusted (intermode, B&W) to convert images to a binary object. Black particles associated with plantlet area were analyzed (size: pixel2: 0-infinity, circularity: 0.00 to 1.00) to determine surface area. Aspect area values were summed to assign an overall area value to each plantlet weekly as a measure of the size and to monitor growth. At the final data reading, area values from plantlets were analyzed to determine overall growth response to treatments (Table 2).
Lepidium ostleri S.L. Welsh and Goodrich plantlets displaying shoot regeneration were analyzed using ImageJ processing software. Plantlets were (A) photographed in a lightbox mounted with two diffused white lights with an object for scale. In ImageJ, images were (B) converted to 8-bit graphics and (C) binary rendered by adjusting threshold. (D) Black particles were analyzed to determine surface area.
Rooting
Plantlets were selected for rooting from the two best shoot organogenesis treatments: T7 (5.0 mg L−1 BAP) and T9 (5.0 mg L−1 BAP, 3.0 mg L−1 IAA) (Table 1). The most vigorous plantlets from each group were separated into 6 treatments (N = 12) using a randomized complete block design with relative plantlet size (small, medium, or large) used as the blocking variable. Priority was given to plantlets that had developed healthy, upright growth and exhibited root development while in vitro. Plantlets showing severe symptoms of hyperhydricity were excluded from the rooting experiment.
Selected plantlets were removed from culture vessels and cleaned of media. Any roots that had developed in vitro were recorded and removed. The plantlets were pulse-treated by immersing their bases into sterile IBA solutions for 5 min each using an aseptic technique (T1: 0 mg L−1, T2: 50.0 mg L−1, T3: 100.0 mg L−1, T4: 200.0 mg L−1, T5: 300.0 mg L−1, and T6: 400.0 mg L−1). Following the pulse treatment, plantlets were immediately transferred to sterile containers holding autoclave-sterilized soil (Agsorb, peat, vermiculite, and sand [1:2:2:1] with 15 g per gallon Osmocote) moistened with ¼ strength MS basal salts. The containers were placed in an incubator at 24 °C with a 16-h photoperiod for 28 d to allow root induction.
Acclimatization
Following root induction, the plants were incubated in their original containers under grow lights. A laminar flow hood was utilized as an incubator to maintain sterility and prevent contamination, which had afflicted previous experimental efforts. With the hood running, lids were removed by increasing increments of time starting with 30-min sessions. Once the plants could tolerate being uncovered for a period of 8 h, they were transferred to well-draining trays filled with sterilized soil and covered with large, translucent lids. Once 12-h increments were tolerated, the lids were removed and the plants were transferred to the greenhouse.
Results and discussion
Shoot proliferation
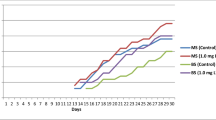
Multiple shoot proliferation was observed on explants in both experimental groups (Fig. 2). Shoot proliferation occurred via indirect somatic embryogenesis with callus formation preceding shoot formation in groups. However, the extent of callus formation varied between all groups before giving rise to shoot formation. For the BAP:Kinetin group, there was a significant difference between treatment groups (N = 249, P = 0.0436) with 59.26% explant shoot response occurring on MS media containing 0.5 mg L−1 BAP (Table 2). Analysis using the Analyze Binary Response for Factorial Design tool in Minitab (2023) was done to determine main and interaction effects of PGRs on response. An ANOVA showed that only BAP had a significant effect on response (P = 0.018; Table 3A) with no significant effects from kinetin or interaction with BAP. Kruskal–Wallis analysis was used due to non-normal distribution of size data. One-way ANOVA showed that only BAP had a significant effect on plant size (P = 0.0058; Table 4).
Response and vigor peaked at the upper limit of BAP concentrations in the BAP:Kinetin group (0.5 mg L−1), which indicated that the organogenic response may continue to rise with an increase of BAP concentration in culture media. The response was also relatively high both in the control (T1) and in treatment 2 containing 0.2 mg L−1 kinetin without the addition of BAP. While BAP has the largest effect on response rates, the interaction between two cytokinin hormones at lower concentrations may be detrimental to optimal growth in L. ostleri. Although previous studies examined Lepidium response to a combination of two cytokinin hormones, it is recognized that both cytokinin and auxin signaling are required for callus and shoot apical meristem development (Schaller et al. 2015). However, there was no interaction effect seen in analysis (Table 3A).
In the proceeding BAP:IAA experiment, the response was still substantially higher at the upper limit of tested BAP concentrations (N = 180, P < 0.001). Interaction effects of PGRs on response could not be analyzed by the same model as the BAP:Kin group due to quasi-complete separation (see ANOVA in Table 3B). Analysis of main effects showed that only BAP had an effect on response frequency (P < 0.001; Table 3B) and on plant size with Kruskal–Wallis tests (P < 0.0001; Table 5). At the highest concentration of BAP (5.0 mg L−1), response declined as IAA concentration increased. While the development of shoot meristems is dependent upon auxin-cytokinin interactions, redirection of auxin transport, and gene expression controlling shoot meristem organization (Motte et al. 2014), shoot response still seemed to be primarily driven by BAP.
The source and developmental conditions of explant tissue may account for notable differences between the two experimental groups. Specifically, the response observed in the BAP:Kinetin control group, which was absent from the BAP:IAA control group, may have resulted from potentially increased endogenous PGRs in the source tissue. Because this tissue was derived from a plant grown in vitro on MS containing BAP and IAA, it is likely that PGRs remained relatively elevated in tissue even after several weeks of culture on plain MS preceding experimental use. In contrast, the source tissue used in the BAP:IAA experiment was not exposed to exogenous PGRs during its development.
Rooting and acclimatization
Approximately 61% of plants across treatment groups survived the rooting and acclimatization process. These plantlets proved to be photosynthetically capable, responded well to transfer from nutrient-rich media to soil, and displayed strong overall root development (Fig. 3). Treatments with 200.0 and 400.0 mg L−1 IBA had the highest survival rates, achieving 83% and 75% survival, respectively. Treatments with 100.0 and 300.0 mg L−1 had the lowest overall survival rates at 33% and 50%, respectively (Table 6). These results suggest that pulse treatment of L. ostleri shoots with 400 mgL−1 IBA was most effective in promoting root development, though no statement of significance can be made using logistic regression due to small treatment size. The control group achieved a 58% survival rate, indicating adequate levels of endogenous phytohormone needed for rooting. It is unclear without further experimentation whether these levels are typical of L. ostleri or are the result of hormone absorption of the in vitro-cultured plant used as an explant source.
Approximately half of the plants utilized for the rooting experiment developed roots in vitro prior to treatment. Although all previously developed roots were removed along with the culture media, 83% of these plants reformed their roots after transfer to soil (Table 6). Alternatively, only 46% of the plants lacking roots in vitro developed roots following treatment and transfer to soil. This observation represents a confounding factor and makes it difficult to determine the full effectiveness of the IBA pulse treatments without further experimentation. This observation also supports the practice of selecting L. ostleri plantlets featuring in vitro root development for the ex vitro rooting phase, regardless of whether these roots are preserved for the rooting phase.
The use of autoclave-sterilized soil and aseptic technique was highly effective in preventing contamination among healthy plantlets during the high-humidity phases of root induction and initial acclimatization. Sterile conditions during initial root induction have been used by other researchers when employing ex vitro rooting approaches (Arya et al. 2003; Shekhawat et al. 2015a; Shekhawat et al. 2015b; Phulwaria et al. 2013; Sharma et al. 2017). These conditions allowed for necessary high humidity levels to be maintained during the 4 wk of root induction and for humidity to be lowered gradually thereafter without microbial proliferation and plant decay.
Plantlets developed in vitro from both treatment groups displayed variation in morphology during shoot and root development. When utilizing plants developed in vitro, it is important to consider the extent of phenotypic variation arising from in vitro regeneration. While differences were observed throughout different experimental phases, no formal assessment was performed to quantify the extent of somaclonal variation. During the acclimatization process, plantlets displayed more typical morphology in leaves, color, and growth habit.
Conclusions
This is the first report of successful micropropagation of L. ostleri using plant tissue culture techniques. The protocol described uses BAP, kinetin, and IAA to induce multiple shoot proliferation on tissue explants with BAP and IAA producing the most robust organogenic response via indirect somatic embryogenesis. Following shoot production, plantlets treated with IBA developed root systems necessary for acclimatization. The use of sterile conditions during the acclimatization phase was highly effective in preventing decay and allowing high overall survival. It took approximately 6 mo to micropropagate L. ostleri with roughly 4 mo to culture and 2 mo to root and harden. The largest challenges encountered in the micropropagation of this species included limited access to healthy source tissue for culturing and contamination prevention following the separation of plantlets from sterile media. Micropropagation of L. ostleri offers an alternative option to seed propagation to produce large quantities of mature plants that would be suitable for various activities, such as cultivation for seed production or experimental out-planting. The development of a micropropagation protocol supports conservation efforts for this rare endemic plant species and contributes knowledge to developing in vitro methods for propagating other Lepidium and rare plant species.
Data Availability
The data that support the findings of this study are available from the corresponding author, [ADN], upon request.
References
Arya V, Shekhawat NS, Singh RP (2003) Micropropagation of Leptadeniareticulata: a medicinal plant. In Vitro Cell Dev Biol – Plant 39:180–185
Bhasin P, Bansil D, Grewal A, Sehrawat AR (2015) Rapid micropropagation of Lepidium sativum L. - a medicinal herb for folklore remedies. J Pharm Res 9:480–483
Bunn E, Turner SR, Dixon KW (2010) Biotechnology for saving rare and threatened flora in a biodiversity hotspot. In Vitro Cell Dev Biol – Plant 47:188–200
Clark NM, Van den Broeck L, Guichard M, Stager A, Tanner HG, Blilou I, Grossmann G, Lyer-Pascuzzi AS, Maizel A, Sparks EE, Sozzani R (2020) Novel imaging modalities shedding light on plant biology: start small and grow big. Ann Rev Plant Biol 71:789–816
Cruz-Cruz CA, Gonzalez-Arnao MT, Engelmann F (2013) Biotechnology and conservation of plant diversity. Resources 2:73–95
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell Dev Biol - Plant 47:5–16
Evenden AG (1998) San Francisco Mountains/Grampian limestone endemics site report. The Nature Conservancy. Unpublished report prepared for the Nature Conservancy, Salt Lake City, UT 12 pp & appendices, pp 1–12. https://www.nature.org/en-us/about-us/where-we-work/united-states/utah/
Hazarika BN, Teixeira da Silva JA, Talukdar A (2006) Effective acclimatization of in vitro cultured plants: methods, physiology and genetics. In: Teixera da Silva JA (ed) Floriculture, ornamental and plant biotechnology: advances and topical issues. Global Science Books 2:427–438
Hill P, Gutierrez B, Carmack L, Kopp OR (2015) Micropropagation of Astragalus holmgreniorum (Holmgren milkvetch), an endemic and endangered species. Plant Cell Tiss Org Cult 121:381–437
Li C, Adhikari R, Yao Y, Miller AG, Kalbaugh K, Li D, Nemali K (2020) Measuring plant growth characteristics using smartphone based image analysis technique in controlled environment agriculture. Comput Electron Agric 168:105123. https://doi.org/10.1016/j.compag.2019.105123
Minitab (2023) Minitab Statistical Software. https://www.minitab.com/en-us/. Cited 28 June 2023
Motte H, Vereecke D, Geelen D, Werbrouck S (2014) The molecular path to in vitro shoot regeneration. Biotech Adv 32:107–121
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Osuna L, Tapia-Perez ME, Figueroa O (2006) Micropropagation of Lepidium virginicum (Brassicaceae), a plant with antiprotozoal activity. In Vitro Cell Dev Biol - Plant 42:596–600
Pande D, Malik S, Boa M, Srivastava PS (2002) A rapid protocol for in vitro micropropagation of Lepidium sativum Linn. and enhancement in the yield of Lepidine. In Vitro Cell Dev Biol 38:451–455
Paunescu A (2009) Biotechnology for endangered plant conservation: a critical overview. Romanian Biotechnol Lett 14:4095–4103
Pence V, Murray S, Whitham L, Cloward D, Barnes H, Van Buren R (2008) Supplementation of the autumn buttercup population in Utah, USA, using in vitro propagated plants. In: Soorae PS (ed) Global re-introduction perspectives, IUCN/SSC Re-introduction Specialist Group, Abu Dhabi, UAE, pp 239–243. https://ser-rrc.org/resource/global-re-introduction-perspecti/
Phulwaria M, Shekhawat NS, Rathore JS, Singh RP (2013) An efficient in vitro regeneration and ex vitro rooting of Ceropegia bulbosa Roxb. - a threatened and pharmaceutical important plant of Indian Thar Desert. Indust Crop Prod 42:25–29
Polzerova H, Greplova M, Frcek J (2011) In vitro multiplication of Lepidium meyenii Walp. J ApplBot Food Qual 84:1–5
Pospisilova J, Ticha I, Kadlecek P, Haisel D, Plzakova S (1999) Acclimatization of micropropagated plants to ex vitro conditions. Biol Plant 42:481–497
Sandhya M, Deepti L, Manisha B, Ravindra M, Shreepad J, Gauri A, Ishani B, Asmit H (2016) Micropropagation of Lepidium sativum. Intl J Life Sci A6:141–144
Sarasan V, Cripps R, Ramsay MM, Atherton C, McMichen M, Prendergrast G, Rowntree JK (2006) Conservation in vitro of threatened plants – progress in the past decade. In Vitro Cell Dev Biol - Plant 42:206–214
Schaller GE, Bishopp A, Kieber JJ (2015) The yin-yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell 37:44–63
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis". Nat Meth 9:671–675
Sharma U, Kataria V, Shekhawat NS (2017) In vitro propagation, ex vitro rooting and leaf micromorphology of Bauhinia racemosa Lam.: a leguminous tree with medicinal values. Physiol Mol Biol Plant 23:969–977
Shekhawat MS, Kannan N, Manokari M, Ravindran CP (2015a) In vitro regeneration of shoots and ex vitro rooting of an important medicinal plant Passiflora foetida L. through nodal segment cultures. J Genet Engineer Biotechnol 13(2):209–214. https://doi.org/10.1016/j.jgeb.2015.08.002
Shekhawat MS, Manokari M, Ravindran CP (2015b) An improved micropropagation protocol by ex vitro rooting of Passiflora edulis Sims. f. flavicarpa Deg. through nodal segment culture. Scientifica. https://doi.org/10.1155/2015/578676
Spalding EP, Miller ND (2013) Image analysis is driving a renaissance in growth measurement. Curr Opin Plant Biol 16:100–104
U.S. Fish and Wildlife Service (USFWS) (2011) Endangered and threatened wildlife and plants; 12-mo finding on a petition to list Astragalus hamiltonii, Penstemon flowersii, Eriogonum soredium, Lepidium ostleri, and Trifolium friscanum as endangered or threatened; rule. Fed Reg 76:10165–10203
Acknowledgements
Funding for the project was provided by the Utah Department of Natural Resources Endangered Species Mitigation Fund (ESMF) and the Utah Valley University Scholarly Activities Committee (SAC). June Perez, Deborah Marchione, Stayner Richards, Cameron Kapp, Stephen Florence, and Michelle Pipegrass of Utah Valley University provided many hours of laboratory assistance critical to the project’s success. Dr. Susan E. Meyer of the U.S. Forest Service Shrub Sciences Laboratory provided invaluable advice and support while helping to facilitate this study.
Funding
The research presented in this paper was in part funded by the Utah Department of Natural Resources Endangered Species Mitigation Fund (award ID 1818).
Author information
Authors and Affiliations
Contributions
AMD, JML, and ORK all participated in various aspects of experimental designs, laboratory work, and preparation of the manuscript. AMD and JML performed statistical analysis. ORK facilitated and supervised all aspects of the research.
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
DeNittis, A.M., Larson, J.M. & Kopp, O.R. Micropropagation of Lepidium ostleri (Brassicaceae), a native endemic plant species. In Vitro Cell.Dev.Biol.-Plant 59, 684–691 (2023). https://doi.org/10.1007/s11627-023-10376-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-023-10376-y