Abstract
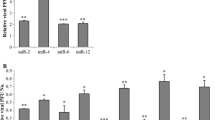
Murine gammaherpesvirus 68 (MHV68) establishes latency mainly in B cells and causes lymphomas reminiscent of human gammaherpesvirus diseases in laboratory mice. To study the molecular mechanism of virus infection and how the viral determinants control cell and eventually cause tumorigenesis, readily available latently infected cell lines are essential. For in vitro MHV68 latency studies, only two cell culture systems have been available. Gammaherpesviruses are known to infect developing B cells and macrophages, therefore we aimed to expand the MHV68 latently infected cell line repertoire. Here, several latently infected immature B cell and macrophage-like cell line clones were generated. Hygromycin-resistant recombinant MHV68 was isolated from a laboratory-made latent cell line, HE2.1, and propagated to develop stable cell lines that carry the viral genome under hygromycin selection. Subclones of these cells lines were analyzed for viral miRNA expression by TaqMan qPCR and assessed for expression of a lytic viral transcript M3. The cell lines maintain the viral genome as an episome shown by the digestion-circularization PCR assay. Latently infected cell lines generated here do not express viral miRNAs higher than the parental cell line. However, these cell lines may provide an alternative tool to study latency mechanisms and miRNA target identification studies.





Similar content being viewed by others
REFERENCES
Thorley-Lawson D.A., Gross A. 2004. Persistence of the Epstein–Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 350, 1328–1337.
Miller G. 1982. Immortalization of human lymphocytes by Epstein−Barr virus. Yale J. Biol. Med. 55, 305–310.
Bechtel J.T., Liang Y., Hvidding J., Ganem D. 2003. Host range of Kaposi’s sarcoma-associated herpesvirus in cultured cells. J. Virol. 77, 6474–6481.
Coleman C.B., Nealy M.S., Tibbetts S.A. 2010. Immature and transitional B cells are latency reservoirs for a gammaherpesvirus. J. Virol. 84, 13045–13052.
Hwang S., Wu T.-T., Tong L.M., Kim K.S., Martinez-Guzman D., Colantonio A.D., Uittenbogaart C.H., Sun R. 2008. Persistent gammaherpesvirus replication and dynamic interaction with the host in vivo. J. Virol. 82, 12498–12509.
Usherwood E.J., Stewart J.P., Nash A.A. 1996. Characterization of tumor cell lines derived from murine gammaherpesvirus-68-infected mice. J. Virol. 70, 6516–6518.
Forrest J.C., Speck S.H. 2008. Establishment of B-cell lines latently infected with reactivation-competent murine gammaherpesvirus 68 provides evidence for viral alteration of a DNA damage-signaling cascade. J. Virol. 82, 7688–7699.
Barton E., Mandal P., Speck S.H. 2011. Pathogenesis and host control of gammaherpesviruses: Lessons from the mouse. Annu. Rev. Immunol. 29, 351–397.
Bartel D.P. 2009. MicroRNAs: Target recognition and regulatory functions. Cell. 136, 215–233.
Kincaid R.P., Sullivan C.S. 2012. Virus-encoded microRNAs: An overview and a look to the future. PLoS Pathog. 8, e1003018.
Cai X., Schäfer A., Lu S., Bilello J.P., Desrosiers R.C., Edwards R., Raab-Traub N., Cullen B.R. 2006. Epstein–Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathog. 2, e23.
Haecker I., Gay L.A., Yang Y., Hu J., Morse A.M., McIntyre L.M., Renne R. 2012. Ago HITS-CLIP expands understanding of Kaposi’s sarcoma-associated herpesvirus miRNA function in primary effusion lymphomas. PLoS Pathog. 8, e1002884.
Ramalingam D., Kieffer-Kwon P., Ziegelbauer J.M. 2012. Emerging themes from EBV and KSHV microRNA targets. Viruses. 4, 1687–1710.
Coleman C.B., McGraw J.E., Feldman E.R., Roth A.N., Keyes L.R., Grau K.R., Cochran S.L., Waldschmidt T.J., Liang C., Forrest J.C., Tibbetts S.A. 2014. A gammaherpesvirus Bcl-2 ortholog blocks B cell receptor-mediated apoptosis and promotes the survival of developing B cells in vivo. PLoS Pathog. 10, e1003916.
Paden C.R., Forrest J.C., Moorman N.J., Speck S.H. 2010. Murine gammaherpesvirus 68 LANA is essential for virus reactivation from splenocytes but not long-term carriage of viral genome. J. Virol. 84, 7214–7224.
Feldman E.R., Kara M., Coleman C.B., Grau K.R., Oko L.M., Krueger B.J., Renne R., van Dyk L.F., Tibbetts S.A. 2014. Virus-encoded microRNAs facilitate gammaherpesvirus latency and pathogenesis in vivo. mBio. 5, e00981-14.
Kara M., Tibbetts S.A. 2021. Empirical validation of overlapping virus lncRNAs and coding transcripts by northern blot. In: Long Non-Coding RNAs in Cancer. Methods in Molecular Biology. Navarro A., Ed. New York: Springer, pp. 243–253.
Hasbold J., Klaus G.G. 1990. Anti-immunoglobulin antibodies induce apoptosis in immature B cell lymphomas. Eur. J. Immunol. 20, 1685–1690.
Flaño E., Husain S.M., Sample J.T., Woodland D.L., Blackman M.A. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165, 1074–1081.
Weck K.E., Kim S.S., Virgin HW I.V., Speck S.H. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73, 3273–3283.
Kim K.J., Kanellopoulos-Langevin C., Merwin R.M., Sachs D.H., Asofsky R. 1979. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J. Immunol. 122, 549–554.
Humme S., Reisbach G., Feederle R., Delecluse H.-J., Bousset K., Hammerschmidt W., Schepers A. 2003. The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proc. Natl. Acad. Sci. U. S. A. 100, 10989–10994.
Hu J., Garber A.C., Renne R. 2002. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 76, 11677–11687.
Forrest J.C., Paden C.R., Allen R.D., Collins J., Speck S.H. 2007. ORF73-null murine gammaherpesvirus 68 reveals roles for mLANA and p53 in virus replication. J. Virol. 81, 11957–11971.
Salinas E., Gupta A., Sifford J.M., Oldenburg D.G., White D.W., Forrest J.C. 2018. Conditional mutagenesis in vivo reveals cell type- and infection stage-specific requirements for LANA in chronic MHV68 infection. PLoS Pathog. 14, e1006865.
Henderson A., Ripley S., Heller M., Kieff E. 1983. Chromosome site for Epstein−Barr virus DNA in a Burkitt tumor cell line and in lymphocytes growth-transformed in vitro. Proc. Natl. Acad. Sci. U. S. A. 80, 1987–1991.
Bullard W.L., Kara M., Gay L.A., Sethuraman S., Wang Y., Nirmalan S., Esemenli A., Feswick A., Hoffman B.A., Renne R., Tibbetts S.A. 2019. Identification of murine gammaherpesvirus 68 miRNA-mRNA hybrids reveals miRNA target conservation among gammaherpesviruses including host translation and protein modification machinery. PLoS Pathog. 15, e1007843.
Gay L.A., Sethuraman S., Thomas M., Turner P.C., Renne R. 2018. Modified cross-linking, ligation, and sequencing of hybrids (qCLASH) identifies Kaposi’s sarcoma-associated herpesvirus microRNA targets in endothelial cells. J. Virol. 92, e02138-17.
Ungerleider N., Bullard W., Kara M., Wang X., Roberts C., Renne R., Tibbetts S., Flemington E.K. 2021. EBV miRNAs are potent effectors of tumor cell transcriptome remodeling in promoting immune escape. PLoS Pathog. 17 (5), e1009217.
Skalsky R.L., Corcoran D.L., Gottwein E., Frank C.L., Kang D., Hafner M., Nusbaum J.D., Feederle R., Delecluse H.J., Luftig M.A., Tuschl T., Ohler U., Cullen B.R. 2012. The viral and cellular microRNA targetome in lymphoblastoid cell lines. PLoS Pathog. 8, e1002484.
Feldman E.R., Tibbetts S.A. 2015. Emerging roles of herpesvirus microRNAs during in vivo infection and pathogenesis. Curr. Pathobiol. Rep. 3, 209–217.
Simas J.P., Swann D., Bowden R., Efstathiou S. 1999. Analysis of murine gammaherpesvirus-68 transcription during lytic and latent infection. J. Gen. Virol. 80, 75–82.
Kara M., O’Grady T., Feldman E.R., Feswick A., Wang Y., Flemington E.K., Tibbetts S.A. 2019. Gammaherpesvirus readthrough transcription generates a long non-coding RNA that is regulated by antisense miRNAs and correlates with enhanced lytic replication in vivo. Noncoding RNA. 5, 6.
Speck S.H., Ganem D. 2010. Viral latency and its regulation: Lessons from the gamma-herpesviruses. Cell Host Microbe. 8, 100–115.
Feederle R., Haar J., Bernhardt K., Linnstaedt S.D., Bannert H., Lips H., Cullen B.R., Delecluse H.-J. 2011. The members of an Epstein-Barr virus microRNA cluster cooperate to transform B lymphocyte. J. Virol. 85, 9801–9810.
Feederle R., Linnstaedt S.D., Bannert H., Lips H., Bencun M., Cullen B.R., Delecluse H.-J. 2011. A viral microRNA cluster strongly potentiates the transforming properties of a human herpesvirus. PLoS Pathog. 7, e1001294.
Gottwein E., Corcoran D.L., Mukherjee N., Skalsky R.L., Hafner M., Nusbaum J.D., Shamulailatpam P., Love C.L., Dave S.S., Tuschl T., Ohler U., Cullen B.R. 2011. Viral microRNA targetome of KSHV-infected primary effusion lymphoma cell lines. Cell Host Microbe. 10, 515–526.
Boss I.W., Nadeau P.E., Abbott J.R., Yang Y., Mergia A., Renne R. 2011. A Kaposi’s sarcoma-associated herpesvirus-encoded ortholog of microRNA miR-155 induces human splenic B-cell expansion in NOD/LtSz-scid IL2Rγnull mice. J. Virol. 85, 9877–9886.
Moody R., Zhu Y., Huang Y., Cui X., Jones T., Bedol-la R., Lei X., Bai Z., Gao S.-J. 2013. KSHV microRNAs mediate cellular transformation and tumorigenesis by redundantly targeting cell growth and survival pathways. PLoS Pathog. 9, e1003857.
Preiss N.K., Kang T., Usherwood Y.-K., Huang Y.H., Branchini B.R., Usherwood E.J. 2020. Control of B cell lymphoma by gammaherpesvirus-induced memory CD8 T cells. J. Immunol. 205, 3372–3382.
Mrázová V., Betáková T., Kúdelová M., Šupolíková M., Lachová V., Lapuníková B., Golais F. 2015. Murine gammaherpesvirus (MHV-68) transforms cultured cells in vitro. Intervirology. 58, 69–72.
Mrázová V., Kúdelová M., Smolinská M., Nováková E., Šupolíková M., Vrbová M., Golais F. 2017. Transformation of cells by photoinactivated murine gamma-herpesvirus 68 during nonproductive and quiescent infection. Intervirology. 60, 61–68.
Sunil-Chandra N.P., Efstathiou S., Nash A.A. 1993. Interactions of murine gammaherpesvirus 68 with B and T cell lines. Virology. 193, 825–833.
Wu T.T., Usherwood E.J., Stewart J.P., Nash A.A., Sun R. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J. Virol. 74, 3659–3667.
Pavlova I.V., Virgin IV H.W., Speck S.H. 2003. Disruption of gammaherpesvirus 68 gene 50 demonstrates that Rta is essential for virus replication. J. Virol. 77, 5731–5739.
Adler H., Messerle M., Wagner M., Koszinowski U.H. 2000. Cloning and mutagenesis of the murine gammaherpesvirus 68 genome as an infectious bacterial artificial chromosome. J. Virol. 74, 6964–6974.
Jain V., Plaisance-Bonstaff K., Sangani R., Lanier C., Dolce A., Hu J., Brulois K., Haecker I., Turner P., Renne R., Krueger B. 2016. A toolbox for herpesvirus miRNA research: Construction of a complete set of KSHV miRNA deletion mutants. Viruses. 8, 54.
Wu T.-T., Liao H.-I., Tong L., Leang R.S., Smith G., Sun R. 2010. Construction and characterization of an infectious murine gammaherpesivrus-68 bacterial artificial chromosome. BioMed. Res. Int. 2011, e926258.
ACKNOWLEDGMENTS
I would like to thank my PhD advisor Prof. Dr. Scott Tibbetts and Tibbetts Lab members. The laboratory work was performed at the University of Florida during my research at Tibbetts Lab.
Funding
The author declared that this study received no specific financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The article does not contain any studies involving humans or animals in experiments performed by the author.
CONFLICT OF INTEREST
The author declare that he has no conflicts of interest.
ADDITIONAL INFORMATION
The author confirmed that the data supporting the findings of this study are available within the supplementary material of the article.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Kara, M. Latent Macrophage and Immature B Cell Lines Generated with Hygromycin-Resistant Murine Gammaherpesvirus 68 Genome Expresses Modest Levels of Viral miRNAs. Mol Biol 58, 123–132 (2024). https://doi.org/10.1134/S0026893324010138
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893324010138