Abstract
Diabetic cardiomyopathy describes heart disease in patients with diabetes who have no other cardiac conditions but have a higher risk of developing heart failure. Specific therapies to treat the diabetic heart are limited. A key mechanism involved in the progression of diabetic cardiomyopathy is dysregulation of cardiac energy metabolism. The aim of this study was to determine if increasing the expression of medium-chain acyl-coenzyme A dehydrogenase (MCAD; encoded by Acadm), a key regulator of fatty acid oxidation, could improve the function of the diabetic heart. Male mice were administered streptozotocin to induce diabetes, which led to diastolic dysfunction 8 weeks post-injection. Mice then received cardiac-selective adeno-associated viral vectors encoding MCAD (rAAV6:MCAD) or control AAV and were followed for 8 weeks. In the non-diabetic heart, rAAV6:MCAD increased MCAD expression (mRNA and protein) and increased Acadl and Acadvl, but an increase in MCAD enzyme activity was not detectable. rAAV6:MCAD delivery in the diabetic heart increased MCAD mRNA expression but did not significantly increase protein, activity, or improve diabetes-induced cardiac pathology or molecular metabolic and lipid markers. The uptake of AAV viral vectors was reduced in the diabetic versus non-diabetic heart, which may have implications for the translation of AAV therapies into the clinic.
Key messages
-
The effects of increasing MCAD in the diabetic heart are unknown.
-
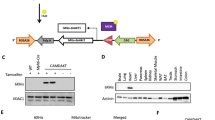
Delivery of rAAV6:MCAD increased MCAD mRNA and protein, but not enzyme activity, in the non-diabetic heart.
-
Independent of MCAD enzyme activity, rAAV6:MCAD increased Acadl and Acadvl in the non-diabetic heart.
-
Increasing MCAD cardiac gene expression alone was not sufficient to protect against diabetes-induced cardiac pathology.
-
AAV transduction efficiency was reduced in the diabetic heart, which has clinical implications.






Similar content being viewed by others
Data availability
Data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Cavallari I, Bhatt DL, Steg PG, Leiter LA, McGuire DK, Mosenzon O, Im K, Raz I, Braunwald E, Scirica BM (2021) Causes and risk factors for death in diabetes: a competing-risk analysis from the SAVOR-TIMI 53 trial. J Am Coll Cardiol 77(14):1837–1840
Kannel WB, Hjortland M, Castelli WP (1974) Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 34(1):29–34
Prakoso D, Tate M, Blasio MJ, Ritchie RH (2021) Adeno-associated viral (AAV) vector-mediated therapeutics for diabetic cardiomyopathy - current and future perspectives. Clin Sci 135(11):1369–1387
Figtree GA, Rådholm K, Barrett TD, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Matthews DR, Shaw W, Neal B (2019) Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes mellitus. Circulation 139(22):2591–2593
Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, McMurray JJV, Solomon SD (2022) SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet 400(10354):757–767
Snaith JR, Greenfield JR (2022) Sodium-glucose cotransporter 2 inhibitors in type 1 diabetes: a missed opportunity for cardiovascular protection? Med J Aust 217(3):126–128
Karwi QG, Sun Q, Lopaschuk GD (2021) The contribution of cardiac fatty acid oxidation to diabetic cardiomyopathy severity. Cells 10(11)
Karwi QG, Zhang L, Wagg CS, Wang W, Ghandi M, Thai D, Yan H, Ussher JR, Oudit GY, Lopaschuk GD (2019) Targeting the glucagon receptor improves cardiac function and enhances insulin sensitivity following a myocardial infarction. Cardiovasc Diabetol 18(1):1
Wang W, Zhang L, Battiprolu PK, Fukushima A, Nguyen K, Milner K, Gupta A, Altamimi T, Byrne N, Mori J, Alrob OA, Wagg C, Fillmore N, Wang S-h, Liu DM, Fu A, Lu JY, Chaves M, Motani A, Ussher JR, Reagan JD, Dyck JRB, Lopaschuk GD (2019) Malonyl CoA decarboxylase inhibition improves cardiac function post-myocardial infarction. JACC: Basic Transl Sci 4(3):385–400
Shao D, Kolwicz SC, Wang P, Roe ND, Villet O, Nishi K, Hsu Y-WA, Flint GV, Caudal A, Wang W, Regnier M, Tian R (2020) Increasing fatty acid oxidation prevents high-fat diet–induced cardiomyopathy through regulating Parkin-mediated mitophagy. Circulation 142(10):983–997
Kolwicz SC Jr, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R (2012) Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res 111(6):728–738
Choi YS, de Mattos ABM, Shao D, Li T, Nabben M, Kim M, Wang W, Tian R, Kolwicz SC Jr (2016) Preservation of myocardial fatty acid oxidation prevents diastolic dysfunction in mice subjected to angiotensin II infusion. J Mol Cell Cardiol 100:64–71
Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H (2001) Metabolic gene expression in fetal and failing human heart. Circulation 104(24):2923–2931
Wiles JR, Leslie N, Knilans TK, Akinbi H (2014) Prolonged QTc interval in association with medium-chain acyl-coenzyme A dehydrogenase deficiency. Pediatrics 133(6):e1781–1786
Marci M, Ajovalasit P (2009) Medium-chain Acyl-CoA dehydrogenase deficiency in an infant with dilated cardiomyopathy. Cardiol Res Pract 2009:281389
Lin RCY, Weeks KL, Gao X-M, Williams RBH, Bernardo BC, Kiriazis H, Matthews VB, Woodcock EA, Bouwman R, Mollica JP, Speirs HJ, Dawes IW, Daly RJ, Shioi T, Izumo S, Febbraio MA, Du X-J, McMullen JR (2010) PI3K(p110a) protects against myocardial infarction-induced heart failure/ Identification of PI3K-regulated miRNAs and mRNAs. Arterioscler Thromb Vasc Biol 30:724–732
Ritchie RH, Love JE, Huynh K, Bernardo BC, Henstridge DC, Kiriazis H, Tham YK, Sapra G, Qin C, Cemerlang N, Boey EJ, Jandeleit-Dahm K, Du XJ, McMullen JR (2012) Enhanced phosphoinositide 3-kinase(p110alpha) activity prevents diabetes-induced cardiomyopathy and superoxide generation in a mouse model of diabetes. Diabetologia 55(12):3369–3381
Saifudeen I, Subhadra L, Konnottil R, Nair RR (2017) Metabolic modulation by medium-chain triglycerides reduces oxidative stress and ameliorates CD36-mediated cardiac remodeling in spontaneously hypertensive rat in the initial and established stages of hypertrophy. J Card Fail 23(3):240–251
Ismael S, Nair RR (2021) Reactivation of fatty acid oxidation by medium chain fatty acid prevents myocyte hypertrophy in H9c2 cell line. Mol Cell Biochem 476(1):483–491
Bernardo BC, Weeks KL, Pongsukwechkul T, Gao X, Kiriazis H, Cemerlang N, Boey EJ, Tham YK, Johnson CJ, Qian H, Du XJ, Gregorevic P, McMullen JR (2018) Gene delivery of medium chain acyl-coenzyme A dehydrogenase (MCAD) induces physiological cardiac hypertrophy and protects against pathological remodelling. Clin Sci 132:381–397
Ho KL, Karwi QG, Connolly D, Pherwani S, Ketema EB, Ussher JR, Lopaschuk GD (2022) Metabolic, structural and biochemical changes in diabetes and the development of heart failure. Diabetologia 65(3):411–423
Li W, Yao M, Wang R, Shi Y, Hou L, Hou Z, Lian K, Zhang N, Wang Y, Li W, Wang W, Jiang L (2018) Profile of cardiac lipid metabolism in STZ-induced diabetic mice. Lipids Health Dis 17(1):231
Bernardo BC, Yildiz GS, Kiriazis H, Harmawan CA, Tai CMK, Ritchie RH, McMullen JR (2022) In vivo inhibition of miR-34a modestly limits cardiac enlargement and fibrosis in a mouse model with established type 1 diabetes-induced cardiomyopathy, but does not improve diastolic function. Cells 11(19):3117
Lother A, Bondareva O, Saadatmand AR, Pollmeier L, Hardtner C, Hilgendorf I, Weichenhan D, Eckstein V, Plass C, Bode C, Backs J, Hein L, Gilsbach R (2021) Diabetes changes gene expression but not DNA methylation in cardiac cells. J Mol Cell Cardiol 151:74–87
Xi Y, Chen D, Dong Z, Lam H, He J, Du K, Chen C, Guo J, Xiao J (2022) RNA sequencing of cardiac in a rat model uncovers potential target LncRNA of diabetic cardiomyopathy. Front Genet 13:848364
Ferreira R, Guerra G, Padrao AI, Melo T, Vitorino R, Duarte JA, Remiao F, Domingues P, Amado F, Domingues MR (2013) Lipidomic characterization of streptozotocin-induced heart mitochondrial dysfunction. Mitochondrion 13(6):762–771
Wang Y, Mohsen AW, Mihalik SJ, Goetzman ES, Vockley J (2010) Evidence for physical association of mitochondrial fatty acid oxidation and oxidative phosphorylation complexes. J Biol Chem 285(39):29834–29841
Zeng J, Li D (2004) Expression and purification of His-tagged rat mitochondrial medium-chain acyl-CoA dehydrogenase wild-type and Arg256 mutant proteins. Protein Expr Purif 37(2):472–478
Bross P, Jensen TG, Andresen BS, Kjeldsen M, Nandy A, Kølvraa S, Ghisla S, Rasched I, Bolund L, Gregersen N (1994) Characterization of wild-type human medium-chain acyl-CoA dehydrogenase (MCAD) and mutant enzymes present in MCAD-deficient patients by two-dimensional gel electrophoresis: evidence for post-translational modification of the enzyme. Biochem Med Metab Biol 52(1):36–44
Course MM, Scott AI, Schoor C, Hsieh CH, Papakyrikos AM, Winter D, Cowan TM, Wang X (2018) Phosphorylation of MCAD selectively rescues PINK1 deficiencies in behavior and metabolism. Mol Biol Cell 29(10):1219–1227
Rennison JH, McElfresh TA, Okere IC, Patel HV, Foster AB, Patel KK, Stoll MS, Minkler PE, Fujioka H, Hoit BD, Young ME, Hoppel CL, Chandler MP (2008) Enhanced acyl-CoA dehydrogenase activity is associated with improved mitochondrial and contractile function in heart failure. Cardiovasc Res 79(2):331–340
Zhong P, Peng J, Liu T, Ding HS (2022) AAV9-mediated cardiac CNTF overexpression exacerbated adverse cardiac remodeling in streptozotocin-induced type 1 diabetic models. Cardiovasc Toxicol 22(1):88–96
Meloni M, Descamps B, Caporali A, Zentilin L, Floris I, Giacca M, Emanueli C (2012) Nerve growth factor gene therapy using adeno-associated viral vectors prevents cardiomyopathy in type 1 diabetic mice. Diabetes 61(1):229–240
Katare R, Caporali A, Zentilin L, Avolio E, Sala-Newby G, Oikawa A, Cesselli D, Beltrami AP, Giacca M, Emanueli C, Madeddu P (2011) Intravenous gene therapy with PIM-1 via a cardiotropic viral vector halts the progression of diabetic cardiomyopathy through promotion of prosurvival signaling. Circ Res 108(10):1238–1251
Tate M, Perera N, Prakoso D, Willis AM, Deo M, Oseghale O, Qian H, Donner DG, Kiriazis H, De Blasio MJ, Gregorevic P, Ritchie RH (2021) Bone morphogenetic protein 7 gene delivery improves cardiac structure and function in a murine model of diabetic cardiomyopathy. Front Pharmacol 12
Prakoso D, De Blasio MJ, Qin C, Rosli S, Kiriazis H, Qian H, Du XJ, Weeks KL, Gregorevic P, McMullen JR, Ritchie RH (2017) Phosphoinositide 3-kinase (p110α) gene delivery limits diabetes-induced cardiac NADPH oxidase and cardiomyopathy in a mouse model with established diastolic dysfunction. Clin Sci (London) 131(12):1345–1360
Prakoso D, Lim SY, Erickson JR, Wallace RS, Lees JG, Tate M, Kiriazis H, Donner DG, Henstridge DC, Davey JR, Qian H, Deo M, Parry LJ, Davidoff AJ, Gregorevic P, Chatham JC, De Blasio MJ, Ritchie RH (2022) Fine-tuning the cardiac O-GlcNAcylation regulatory enzymes governs the functional and structural phenotype of the diabetic heart. Cardiovasc Res 118(1):212–225
Prakoso D, De Blasio MJ, Tate M, Kiriazis H, Donner DG, Qian H, Nash D, Deo M, Weeks KL, Parry LJ, Gregorevic P, McMullen JR, Ritchie RH (2020) Gene therapy targeting cardiac phosphoinositide 3-kinase (p110alpha) attenuates cardiac remodeling in type 2 diabetes. Am J Physiol Heart Circ Physiol 318(4):H840–H852
Kibel A, Selthofer-Relatic K, Drenjancevic I, Bacun T, Bosnjak I, Kibel D, Gros M (2017) Coronary microvascular dysfunction in diabetes mellitus. J Int Med Res 45(6):1901–1929
Chang SC, Ren S, Rau CD, Wang JJ (2018) Isoproterenol-induced heart failure mouse model using osmotic pump implantation. Methods Mol Biol 1816:207–220
Madla CM, Gavins FKH, Merchant HA, Orlu M, Murdan S, Basit AW (2021) Let’s talk about sex: Differences in drug therapy in males and females. Adv Drug Deliv Rev 175:113804
Chang DH, Dumanski SM, Ahmed SB (2023) Female sex-specific considerations to improve rigor and reproducibility in cardiovascular research. Am J Physiol Heart Circ Physiol 324(3):H279–h287
Toedebusch R, Belenchia A, Pulakat L (2018) Diabetic cardiomyopathy: impact of biological sex on disease development and molecular signatures. Front Physiol 9:453
Kannel WB, McGee DL (1979) Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care 2(2):120–126
Huxley R, Barzi F, Woodward M (2006) Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 332(7533):73–78
Suys BE, Katier N, Rooman RP, Matthys D, Op De Beeck L, Du Caju MV, De Wolf D (2004) Female children and adolescents with type 1 diabetes have more pronounced early echocardiographic signs of diabetic cardiomyopathy. Diabetes Care 27(8):1947–1953
Saadane A, Lessieur EM, Du Y, Liu H, Kern TS (2020) Successful induction of diabetes in mice demonstrates no gender difference in development of early diabetic retinopathy. PLoS ONE 15(9):e0238727
Chandramouli C, Reichelt ME, Curl CL, Varma U, Bienvenu LA, Koutsifeli P, Raaijmakers AJA, De Blasio MJ, Qin CX, Jenkins AJ, Ritchie RH, Mellor KM, Delbridge LMD (2018) Diastolic dysfunction is more apparent in STZ-induced diabetic female mice, despite less pronounced hyperglycemia. Sci Rep 8(1):2346
Ritterhoff J, McMillen TS, Villet O, Young S, Kolwicz SC Jr, Senn T, Caudal A, Tian R (2021) Increasing fatty acid oxidation elicits a sex-dependent response in failing mouse hearts. J Mol Cell Cardiol 158:1–10
Zhao Z, Anselmo AC, Mitragotri S (2022) Viral vector-based gene therapies in the clinic. Bioeng Transl Med 7(1):e10258
Tanne JH (2022) FDA approves $3.5m gene therapy for adults with haemophilia B. BMJ 379:o2858
Katz MG, Fargnoli AS, Yarnall C, Perez A, Isidro A, Hajjar RJ, Bridges CR (2018) Technique of complete heart isolation with continuous cardiac perfusion during cardiopulmonary bypass: new opportunities for gene therapy. J Extra Corpor Technol 50(3):193–198
Liu YB, Xu BC, Chen YT, Yuan X, Liu JY, Liu T, Du GZ, Jiang W, Yang Y, Zhu Y, Chen LJ, Ding BS, Wei YQ, Yang L (2021) Directed evolution of AAV accounting for long-term and enhanced transduction of cardiovascular endothelial cells. Mol Ther Methods Clin Dev 22:148–161
Acknowledgements
We wish to thank Ms Stephanie Jansen (Alfred Research Alliance Animal Facility) for performing the AAV tail vein injections, Ms Amy Hsu (Baker Heart and Diabetes Institute) for technical assistance, and Dr Hongwei Qian (University of Melbourne) for producing the AAV vectors.
Funding
This work was supported by a grant to B.C.B and K.L.W from the Diabetes Australia Research Program (Y19G-BERB), Women in Science Grants from the Baker Heart and Diabetes Institute (to B.C.B), and the Victorian Government’s Operational Infrastructure Support Program. B.C.B is supported by a Baker Fellowship (Baker Heart and Diabetes Institute, Australia). K.L.W is supported by a Future Leader Fellowship from the National Heart Foundation of Australia (award ID 102539). N.M.S. is supported by a Research Training Program scholarship from Monash University. B.G.D, P.G, L.M.D.D, and J.R.M are supported by funding from the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Contributions
Conceptualization: B.C.B, K.L.W; methodology: B.C.B, K.L.W; investigation: B.C.B, K.L.W, H.K, G.D.W, E.I.M, N.M.S, A.J.A.R, C.A.H, A.J.T, G.S.Y, Y.L; formal analysis: B.C.B, K.L.W, H.K, G.D.W, E.I.M, N.M.S, A.J.A.R, A.J.T; writing—original draft preparation: K.L.W, B.C.B; writing—review and editing: K.L.W, H.K, G.D.W, A.J.T, P.G, L.M.D.D, J.R.M, B.C.B; funding acquisition: B.C.B, K.L.W; resources: G.D.W, B.G.D, P.G, L.M.D.D, J.R.M; supervision: B.C.B, K.L.W, J.R.M. project administration: B.C.B. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study does not involve human subjects. All experiments using animals were conducted in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes (National Health & Medical Research Council of Australia, 8th Edition, 2013). All animal procedures and care were approved by the Alfred Research Alliance Animal Ethics Committee (Animal Ethics Committee Approved Project Number E/1867/2018/B).
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kate L. Weeks, Julie R. McMullen, and Bianca C. Bernardo are equal senior authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weeks, K.L., Kiriazis, H., Wadley, G.D. et al. A gene therapy targeting medium-chain acyl-CoA dehydrogenase (MCAD) did not protect against diabetes-induced cardiac pathology. J Mol Med 102, 95–111 (2024). https://doi.org/10.1007/s00109-023-02397-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02397-2