Abstract
The greenhouse gas (GHG) emissions of spring cereal monoculture under long-term conventional tillage (CT) and no-till (NT) treatment established in 2018 were measured in a peatland in Southwestern Finland during the period 2018–2021. Nitrous oxide (N2O), carbon dioxide (CO2) and methane (CH4) fluxes were measured with chambers approximately every two weeks throughout the period under study. Net ecosystem exchange was measured during the growing seasons, and hourly ecosystem respiration (ER) and gross photosynthesis (GP) were modelled with empirical models. Across the whole period, annual emissions were 6.8 ± 1.2 and 5.7 ± 1.2 Mg CO2–C ha −1 yr−1 (net ecosystem carbon balance), 8.8 ± 2.0 and 7.1 ± 2.0 kg N2O–N ha−1 yr−1, and − 0.43 ± 0.31 and − 0.40 ± 0.31 kg CH4-C ha−1 yr−1 for CT and NT, respectively. The global warming potential was lower in NT (p = 0.045), and it ranged from 26 to 34 Mg CO2 eq. ha−1 yr−1 in CT and from 19 to 31 Mg CO2 eq. ha−1 yr−1 in NT. The management effect on the rates of single GHGs was not consistent over the years. Higher GP was found in CT in 2019 and in NT in 2020. Differences in ER between treatments occurred mostly outside the growing season, especially after ploughing, but the annual rates did not differ statistically. NT reduced the N2O emissions by 31% compared to CT in 2020 (p = 0.044) while there were no differences between the treatments in other years. The results indicate that NT may have potential to reduce slightly CO2 and N2O emissions from cultivated peat soil, but the results originate from the first three years after a management change from CT to NT, and there is still a lack of long-term results on NT on cultivated peat soils.
Similar content being viewed by others
Introduction
As a large reservoir of organic carbon, peatlands have a key role in climate regulation (Nichols and Peteet 2019). Pristine peatlands act as a carbon sink but drainage for forestry or agriculture leads to peat oxidation and decomposition changing them into net greenhouse gas (GHG) emission sources (Joosten et al. 2016). Although they account for only 3% of global land area, cultivated peatlands and organic soils contribute 8% of agricultural GHG emissions globally (FAO 2020). In drained peatlands, CO2 emissions constitute the majority of the climate impact, followed by N2O emissions (Tan et al. 2020) whereas CH4 fluxes have a minor role as a source or sink of carbon (Maljanen et al. 2010; Kandel et al. 2018).
To mitigate GHG emissions from drained peatlands, rewetting is considered the most effective and primary management practice (Humpenöder et al. 2020; EC 2021). However, rewetting does not provide income to landowners on a large scale before there are abundant funds for rewetting or functional value chains for paludiculture (Ziegler 2020). Due to such socio-economic barriers, interim management practices that slow peat decomposition rates are needed in the short term to complement rewetting efforts.
In no-till management (NT), seeds are directly sown in the stubble without annual ploughing and harrowing. As this reduces disturbance of the soil surface compared to conventional tillage (CT), NT might also reduce peat decomposition and the related GHG emissions. NT brings immediate savings in workload and fuel usage (West and Marland 2002) and reduces erosion in mineral soils (Honkanen et al. 2021) but its role in carbon sequestration and reducing GHG emissions is less straightforward. It has been commonly found that NT increases the carbon content in the topsoil of mineral soils but the potential in climate change mitigation is limited considering the whole soil profile (Ogle et al. 2019; Cai et al. 2022). The effects of NT on the emissions of N2O are similarly variable with some indication of benefits from long-term NT management (Van Kessel et al. 2013).
The physical, chemical and biological properties of the soil as well as residue placement differ between NT and CT soils with potential implications for the microbial processes responsible for GHG emissions. NT increases topsoil density and reduces porosity compared to CT (Elder and Lal 2008b) which might contribute to slower gas exchange (Maljanen et al. 2003b) and reduced emissions in organic soils. Denser topsoil can also restrict oxygen diffusion and favour denitrification leading to enhanced N2O emissions (Ball et al. 1999). There may be different temperature and moisture regimes in the treatments leading to different timing or levels of emission peaks for example due to different timing of frost melt in the spring (Regina and Alakukku 2010). Crop residues are not well mixed in the topsoil in NT leading to slower decomposition and different stratification of biological activity and released nutrients (Mutegi et al. 2010). However, organic soils may respond distinctly to NT compared to mineral soils. Long-term measurements of relevant GHGs are needed to elucidate the full impacts of adopting NT on cultivated organic soil GHG dynamics.
Although several meta-analyses have been performed on the effects of NT on GHG emissions in mineral soils (Ogle et al. 2012; Van Kessel et al. 2013; Shakoor et al. 2021) such data compilations from organic soils are lacking as only a couple of studies on GHG emissions in NT have been performed on organic soils. Elder and Lal (2008b) reported lower N2O emissions in NT but no significant difference in CO2 emissions compared to CT in an organic soil. In the study by Regina and Alakukku (2010), NT tended to have lower CO2 emissions and N2O emissions compared to CT at several Finnish sites, including one that was on organic soil.
Both the above-mentioned studies reported CO2 measurements from ecosystem respiration, not the net ecosystem carbon balance. To better understand carbon dynamics in peatlands, it is necessary to consider both soil and plant contributions. Compared to mineral soils, traditional methods of measuring soil carbon stock changes are less effective in peatlands due to the rapid loss of topsoil, which limits comparability of repeated samplings (Heikkinen et al. 2013). The aim of this study was to quantify the climatic effects of tillage managements in an arable field on organic soil. A three-year experiment with CT and NT treatments was set up in Southwestern Finland for measuring carbon exchange and emissions of N2O and CH4. We hypothesised that reducing tillage would decrease losses of carbon in soil respiration due to less soil disturbance and increase N2O emissions due to a denser topsoil layer compared to conventionally tilled soil.
Materials and methods
Study site
The experimental field was located in Kuuma, Jokioinen in Southwestern Finland (60.22 °N, 24.78 °E, 110 m a.s.l.). The climate is boreal humid with a long-term (1991–2021) annual mean temperature of 5.2 °C and precipitation of 621 mm (Jokinen et al. 2021). The total annual global radiation is 3358 MJ m−2, and the total sunshine duration is 1699 h. Typically, the soil is frozen and covered with snow from December to March. The field was a highly composed fen with organic carbon content of 25% and pH 5.5 in the surface layer (0–20 cm) and peat depth ranging from 0.8 to over 2 m. It had been drained and cultivated at least from the end of the 19th century. The original subsurface drainage system with tile drains was replaced by modern plastic pipes embedded in gravel in the 1960s. The distance between the pipes was 18 m until 1979 when it was changed to 9 m. The drainage depth was 60–80 cm during the experiment. Prior to the beginning of this experiment, the field was monocultured with spring cereals for several years. These were conventionally cultivated, and the field was ploughed with typical farming machinery.
The experimental site was placed between the original subsurface drainage pipes so that each plot (8 × 18 m = 144 m2) had hydrologically as similar conditions as possible (Fig. 1). The experimental setup was a randomised split-plot experiment in four blocks. The field was set up with four treatments: half of the CT and NT plots were sown with a cover crop (CC) (Italian ryegrass, Lolium multiflorum). Thus, the resulting treatments were CT, NT, CT + CC and NT + CC. The initial treatment CT was continued as a control in the plots assigned to it, and the other treatments were introduced as new treatments.
Experimental set-up of 16 plots in four blocks located in Jokioinen, Southwestern Finland. Plots were placed between the original sub-surface drainage pipes and the additional pipes were in the middle of the plots. Conventionally ploughed plots (CT) are marked in white and no-tilled (NT) plots are marked in grey. Squares and small circles are denoting installed chamber collars and bare soil collars in each plot. The locations of the soil temperature loggers are marked in the plots 5 and 6. Water table depth (WTD) was measured at four points on the edges of the field. Cover crop was sown to plots with underlined number. The figure is not in scale
The whole field was ploughed in the spring of 2017 and was cultivated with oats using common cultivation practices. The experiment was set up on 24 May 2018, and the dates for the most important cultivation practices during the experiment are given in Table 1.
The experimental field was cultivated with oats (Avena sativa) in 2018, barley (Hordeum vulgare) in 2019 and oats again in 2020. The sowing density was 500 seeds m−2 for both barley and oats. The plots were fertilised each year with Yara Mila Y3 fertiliser which contains 26% nitrogen (N), 3% phosphorus (P) and 8% potassium (K). The applied amount was 260 kg ha−1 each year which corresponds to N rate of about 60 kg per hectare. The fertiliser was given once a year at the time of sowing. Herbicides such as Premium Classic, FMC Agricultural Solutions A/S, USA (12 g ha−1), Starane, Corteva Agriscience, USA (0.25 l ha−1), Refine Super SX, FMC Agricultural Solutions A/S, USA (0.25 g ha−1) and Primus, Corteva Agriscience, USA (0.06 l ha−1) were applied once a year to control weeds, with the same rates and frequencies in all treatments.
Ploughing, sowing, plant protection, fertilising and harvesting were done using typical farming machinery. Sowing was performed using a VM300SK direct sowing machine (Vieskan Metalli Oy, Finland) in 2019–2020 and Väderstad 300–400 C/S (Väderstad Ab, Sweden) in 2018. The CT plots were mouldboard ploughed to a depth of 20 cm. Plot grain yields were determined in the middle of the plots with an experimental combine harvester (Sampo Rosenlew SR2010). Yield outside of these areas was harvested with a field-scale combine harvester.
Soil samples for basic soil analyses were taken from the 0–20 cm layer of each plot in spring 2018 using an auger (diameter 3 cm; approx. 20 subsamples were pooled to make up one sample). Soil core samples for dry bulk density and porosity (diameter 5 cm) were taken from the surface layer (0–17.5 cm) of each plot in Oct 2020 using the Kopec corer, and the samples were dried at 37 °C for a week. Soil conductivity and acidity were determined using the ISO 11,265 and ISO 10,390 methods, respectively. Nutrient content was analysed as described in Vuorinen and Mäkitie (1955). Soil carbon and nitrogen were determined using the dry combustion method (Leco TruMac CN Determinator, Leco Corp. St. Joseph, MI, USA).
Flux measurements
Closed opaque chambers were used to measure ecosystem respiration (ER) and N2O and CH4 fluxes. In each plot, a 60 cm × 60 cm steel collar was installed at a depth of 10–15 cm. The location of the collars was 2–3 m from the edge of the plot and 4 m from the edges of the adjacent plots (Fig. 1). The collars were removed before sowing, harvesting and ploughing. An aluminium chamber (height 40 cm) mounted at the top of the collar was sealed with water in the groove of the upper edge of the collar. The clear aluminium surface effectively reflected light and kept the temperature change moderate inside the chamber. Steel extensions were used when the height of the crop exceeded the height of the chamber. The measurements were done during daytime between 10 am and 2 pm approximately biweekly, or monthly in the winter. The chambers were closed for 30 min, and four 20 ml gas samples were taken with a 60-ml plastic syringe to pre-vacuumed vials (Exetainer, Labco Limited, UK) in 10-minute intervals starting immediately after closing. Prior to sampling, the syringe was pumped five times to mix the air in the chamber. The samples were analysed with a gas chromatograph (Agilent 7890 Agilent Technologies, Inc., Wilmington, DE, USA) equipped with flame ionizer and electron capture detectors, and a nickel catalyst for converting CO2 to CH4. The gas chromatograph had a 2 ml sample loop and a backflush system for separating water from the sample and flushing the precolumn between the runs. The precolumn and analytical columns consisted of 1.8 and 3 m long steel columns, respectively, and were packed with 80/100 mesh Hayesep Q (Supelco Inc., Bellefonte, PA, USA). Nitrogen was used as the carrier gas and a standard gas mixture of known concentration of CO2, N2O and CH4 was used for a calibration curve with seven concentration points. The detection limit for N2O was 1.2 µg N m−2 h−1. An autosampler (222 XL Liquid handler, Gilson Medical Electronics, France) fed the samples to the loop of the gas chromatograph. A linear regression model was fitted to calculate gas concentrations and the ideal gas law was used to solve the flux rate for every enclosure. The criterion of R2 > 0.9 for CO2 was used as the acceptance limit for all gases.
A transparent chamber (60 × 60 × 60 cm) made of polycarbonate plexiglass (1 mm, light transmission 95%) was used to measure NEE approximately biweekly during the growing season. The chamber was equipped with a Vaisala GMP-343 probe and a temperature and humidity sensor (Vaisala Oy, Vantaa, Finland) and two fans for mixing the air during the measurement. One or two layers of a white fabric shroud and one blackout curtain were used to control the amount of light entering the chamber (approximately 100%, 50%, 25%, 0% of ambient radiation). The measurements were done in the same collars as the opaque chamber measurements. Four measurements with different amount of entering light were taken from each subplot in order to cover a large range of light conditions during one measurement round to increase the number of available data points for the light response curve of photosynthesis. Each measurement took one minute with a five second sampling rate. The chamber was flushed after each measurement to reconstitute ambient CO2 and air humidity contents. A lag time of 10 s was applied after closing and before starting the measurement to exclude the deadband when the flux was not yet stabilised. Clear sky conditions were preferred to avoid problems related to changing cloud cover and to achieve the widest possible range of available light. On the hottest summer days, freezer blocks were used to cool the chamber air. The temperature change inside the chamber was less than 1.5 degrees which was also used as a criterion for data filtering.
Due to the short chamber enclosure time, the change in gas concentration during the chamber enclosure was mostly linear. The primary measurement results as parts per million (ppm) unit were converted to g m−2 h−1 in accordance with the ideal gas law using the measured temperature inside the chamber.
If the snow cover was > 20 cm, a concentration snow gradient method as in Maljanen et al. (2003a) was used to determine the GHG fluxes. A probe made of a steel pipe (Ø 3 mm), with a three-way valve and a plastic syringe, was used to sample 15 ml of air just above the snow cover, at the bottom of the snow cover and at one depth in between in three replicates per plot. The gas was stored in the pre-vacuumed vials and the concentrations were determined gas chromatographically as with the opaque chamber samples. The fluxes were calculated using the equation based on Fick’s law as in Maljanen et al. (2003a).
Measurements for bare soil respiration were made in 7/2019–5/2021. We installed one steel air ventilation pipe 27 cm in diameter and 30 cm in length to the depth of 5–10 cm in each of the 16 subplots next to the opaque chamber collars (Fig. 1). All green vegetation within the chamber area was removed. For the measurements, the cylinders were closed with a cover equipped with a CO2 sensor (GMP-343; Vaisala Oyj, Vantaa, Finland) and a small fan. One measurement lasted for one minute with a five second sampling rate, and was taken about once a week or two, more frequently in summer than in winter. Measurements were used to estimate the total soil respiration in the growing seasons of 2019 and 2020. The measurement frequency was raised to determine the effect of ploughing on soil respiration in autumn 2020 when the respiration was measured 0, 30, 90 and 210 min after ploughing as well as two times the next day and once on the third day after ploughing. The additional carbon loss caused by ploughing was also included in the estimate of total annual respiration when determining the ER.
Ancillary measurements
Photosynthetically active radiation (PAR) was continuously measured at the edge of the field by LI-190 quantum PAR sensor (LI-COR, Lincoln, Nebraska, USA) with a one minute sampling rate. Soil temperature was measured first at the depth of 10 cm (but at the depth of 5 cm from May 2020 on to achieve better response of CO2 to air temperature) in CT and NT plots (one plot per treatment) with Elcolog sensors (Elcoplast Oy, Tampere, Finland). Two sensors were installed in both plots with a sampling rate of one hour in summer and 2.5 h in winter. WTD was measured from four monitoring pipes at the edges of the experimental field at the time of the opaque chamber measurements. The air temperature, precipitation and radiation data were taken from the weather station of the Finnish Meteorological Institute (FMI, CC BY 4.0) located about 10 km from the site. Gaps in the measured PAR data were filled with global radiation data from FMI and corrected using the ratio of global radiation and the measured PAR.
Leaf area index (LAI) was measured at the same time with the transparent chamber measurements with a portable LAI meter (SunScan; Delta-T Devices Ltd, Cambridge, United Kingdom). Because the measured LAI corresponds poorly to the actual photosynthetically active biomass in the late growing season due to the ripening of cereals, we selected the highest measured LAI values for the beginning of August and assumed that LAI is zero in the mid-September about a week before harvesting when the measured GP was negligible. LAI of the CC in the respective treatments was set to zero after ploughing in the CT plots and at mid-December in the NT plots. LAI values > 3 were set to 3 as they were assumed not to affect GP due to saturation of the reflectance (Aparicio et al. 2000).
Flux modelling
NEE (net ecosystem exchange) consists of GP (gross photosynthesis) and ER (ecosystem respiration) and thus GP was estimated for each NEE measurement by (Eq. 1),
where the full darkened transparent chamber measurement result (ER) is subtracted from the light-dependent flux (NEE) measured during the same day. Thus, we follow the sign convention with positive ER and negative GP values. The light response of GP was estimated for individual plots and measurement days (four measurements per plot at 0%, 25%, 50% and 100% of ambient PAR) with a rectangular hyperbolic saturation curve (Thornley and Johnson 1990):
where α (g CO2 µmol per photons in hour) is the initial slope of the photosynthetic light response and GPmax (g CO2 m−2 h−1) is the maximum value of GP at infinite PAR. Estimated α and GPmax were used to predict GP at PAR = 1000 µmol photon m−2 s−1 for individual plots and measurement days to compare differences between management methods.
Annual ER and GP (May to April) were modelled using nonlinear regression (fitnlm function in Matlab) for all 16 plots and all years separately summing up to 96 models in total. Empirical models were used for ER as in Lohila et al. (2003) and for GP as in Kandel et al. (2013). Instead of the phytomass indices used in the above publications, we used LAI to describe the stage of the crop growth. Air temperature and PAR were assumed to be the same for all plots, whereas we used the measured LAI for each plot distinctly and the soil temperature from either CT or NT plots.
We used the following equation defined by Long et al. (1993) for GP to estimate empirical coefficients (Amax and k):
where PAR is the measured photosynthetically active radiation, LAI is the measured leaf area index, Amax is the asymptotic maximum, and k is a half-saturation value. TScale represents the temperature sensitivity of photosynthesis and follows the equation performed by (Raich et al. 1991):
where T is the measured temperature, photosynthetically active minimum temperature Tmin is − 2 °C, maximum Tmax is 40 °C and the optimum is 20 °C as in (Kandel et al. 2013).
ER consists of autotrophic (Rauto) i.e. plant respiration and heterotrophic (Rhetero) i.e. soil respiration (Lloyd and Taylor 1994):
where Tsoil is the measured soil temperature, LAI is the measured leaf area index, Tair is the measured air temperature, R0s is soil respiration at the reference temperature 10 °C, R0p is plant respiration at the reference temperature at 10 °C, E0s is ecosystem sensitivity set to 308 and bd was the temperature dependence of dark respiration set to 5000 as in (Lohila et al. 2003).
The empirical coefficients (R0p and bd) were estimated with a nonlinear regression model similarly as in the case of GP. Hourly timeseries of GP and ER were predicted with the above equations using the modelled parameters and hourly timeseries of the field measurements. Hourly time points for LAI, soil temperature and PAR were acquired from the measured values by linear interpolation. Gaps in soil temperature during sowing, harvesting or ploughing were filled with the averaged air temperature using the daily moving mean which correlated with soil temperature in the full dataset (R2 = 0.8). The NEE was calculated by subtracting the modelled GP from the modelled ER. ER was estimated using data from both opaque chambers and darkened transparent chambers. Annual fluxes were computed as integral of the hourly fluxes with a trapezoidal method (trapz function in Matlab). Net ecosystem carbon balance consists of NEE and the amount of carbon removed in yield. The carbon content of the crop yield was assumed to be 45% (Jensen et al. 2005).
Hourly soil respiration was modelled for both treatments for the periods between sowing and harvesting in 2019 and 2020 based on all bare soil respiration measurements and timeseries of soil temperature. Procedure for flux fitting was the same as for the transparent chamber measurements but in the modelling phase only Eq. 6 was used to estimate the parameter R0S, and the models were built up for both treatments instead of each plot. Total effluxes were accounted for the growing seasons (between sowing and harvesting) of 2019 and 2020.
The peak caused by ploughing was determined for each plot by linearly interpolating the interval between each measurement starting immediately after ploughing and ending with measurements on the third day. The cumulated three-day emission amount according to the flux measurements before ploughing was subtracted from the calculated peak emissions to estimate the increase in soil respiration caused by tillage.
Data cleaning and analysis
Some outliers were identified at the beginning of the measurement if the flux was not yet stabilized due to fluctuations or the slow response of the measuring device. Obvious outliers from the beginning (first three data points) of the transparent chamber flux measurement were removed with Matlab’s isoutlier command. The outlier was defined to be more than three scaled median absolute deviations (MAD) from the median of the linear regression standardized residuals, and this resulted in the removal of 162 data points out of 18 000 in 1533 flux measurements. For the transparent chamber measurements, the criteria R2 > 0.9 for the fitted linear assumption of flux measurements would exclude a large amount of data, especially with a small change in CO2, leading to a biased dataset. Therefore, we decided to add the criterion Sxy < 3.0 g m−2 h−1 as in Kutzbach et al. (2007) (Sxy is the standard deviation of the residuals and 3.0 g m−2 h−1 is the 95% percentile of measurements). This procedure resulted in the removal of 97 values out of total 1533 measurements. In the modelling phase, fitted values were examined, and outliers were removed to avoid distortion. Outliers were defined as observations with an absolute value of standardised residuals greater than three. In 2018, 8 out of 411 GP values and 14 of 400 ER values were removed. In 2019, 11 out of 529 GP values and 7 of 433 ER values were removed. In the dataset of 2020, 13 out of 496 GP values and 29 of 494 ER values were removed. The model’s estimated parameters Amax, k of GP, R0s and R0p of ER and model correlations are shown in Table 2. The measured versus model predicted values of GP and ER are shown by managements and years in Fig. 2.
Comparisons between the measured and the model predicted values (g CO2 m−2 h−1) of gross photosynthesis (GP) and ecosystem respiration (ER) in 2018-2020. Outliers are excluded. Black lines represent the 1:1 line and the red and blue lines linear regression between the measured and modelled values for conventional tillage (CT) and no-till (NT)
For the bare soil fluxes, the criterion R2 > 0.9 was used for data cleaning which resulted in the removal of 86 values out of 775. The measurement results during the intensive period immediately after ploughing (n = 70) were also removed from the modelling data. Outliers (n = 5) in the models were processed by removing measurements whose standardised residuals exceeded the threshold value of 3.
For opaque chamber measurements, data points that did not pass the criterion CO2 R2 > 0.9 (26 out of total 928 datapoints), were removed. For N2O and CH4, the removed data points were replaced with the treatment average at that measurement time. A separate cumulative annual sum was created for each plot (trapezoidal sum, cumtrapz in Matlab). The beginning of the time series (21 days) before the first measurement in 2018, was extrapolated based on the first measurement (11.6) by assuming that CH4 and N2O levels remain the same. The same was done at the end of the time series in 2020, where there were 9 days to extrapolate. All the data cleaning and processing was done with Matlab (The Math Works, Inc., MATLAB, version 2019b).
For the global warming potential over one hundred years (GWP-100), the coefficients for converting N2O and CH4 into CO2 equivalents were 273 and 27, respectively (Forster et al. 2021).
Linear mixed models with management (CT and NT), cover crop (with and without), year (2018, 2019 and 2020), and all their interactions were denoted as fixed effects. Block and block × year were assumed to be independent and normally distributed random effects. Correlation between years was taken into account using a heterogeneous or homogeneous compound symmetry (CSH and CS) or unstructured covariance structure. The latter does not impose any specific constraints on the relationships between the observations, while the others have constant covariance. CSH also allows non-constant variance, while CS has constant variance. The most suitable covariance structure was chosen using Akaike’s Information Criterion (AICc).
The models were fitted using the residual maximum likelihood (REML) method and degrees of freedom were estimated using the Kenward-Roger method. The residuals were plotted against the fitted values and the normality of the residuals were checked using boxplots. The method of Tukey-Kramer was used for all pairwise comparisons of means with a significance level of 0.05. All statistical analyses were performed using the GLIMMIX procedure in the SAS Enterprise Guide 8.3 (SAS Institute Inc., Cary, NC, USA).
Results
Site variables
Annual mean temperature (May–Apr) was 6.8 °C in 2018, 6.7 °C in 2019 and 6.0 °C in 2020. These values were thus higher than the long-term average of 5.2 °C during the reference period 1991–2020 (Jokinen et al. 2021). Precipitation for the three consecutive years was 471 mm, 788 mm and 596 mm, respectively, exhibiting both lower and higher values compared to the long-term average of 621 mm during the reference period. The soil is typically frozen and covered by snow at this location during the winter but winter 2019–2020 was warm without a long period of snow cover and frost. Between sowing and harvest, the global radiation sum (kWh m−2) was 430, 610 and 480 in 2018, 2019 and 2020, respectively.
On average, WTD was 68 ± 12 cm ranging from 29 to 99 cm. Highest WDTs were observed after the snow melt, on 4 Apr 2019, 11 Feb 2020 and 30 Mar 2021 and lowest on 23 Jul 2018, 11 Sep 2019 and 14 Oct 2021. The peat layer was deep (ranging from 0.8 to over 2 m), and its organic carbon content was about 25% in the topsoil 0–20 cm, 29% in the 20–40 cm layer, and 35% in the 40–60 cm layer. The topsoil properties are presented in Table 3. The peat was highly decomposed, as indicated by the low carbon content, high ash content and von Post value of eight. No significant differences in dry bulk density or porosity were observed between treatments in Oct 2020, after three growing seasons of differing soil management.
Crop yields did not differ significantly between treatments, but barley (2019) had higher yields compared to oats. On average, the dry yields (kg ha−1) were 3100 ± 300, 4400 ± 600 and 3200 ± 300 in 2018, 2019 and 2020, respectively. LAI was at its highest in the end of July, and the maximum peak in LAI values was around 9 in 2019 and 2020 but 4–5 in 2018. LAI of the CC was about 0.5.
The CC yield was low (mean above ground yield 410 ± 300 kg dry matter ha−1) and it did not cause any clear differences between treatments except for the estimated annual ER (Table 4). The LAI of the CC was included in the models and calculations for GHG fluxes.
CO2 fluxes
The time series of GP(1000) value predicted by the model are shown in Fig. 3. There were data points with low model R2 (< 0.7) especially at the end of the growing season when GP was negligible, and such data points were removed from Fig. 3 (78 values out of 371). GP(1000) was at its highest in the late summer with the peak values being on 1 Aug in 2018, 17 Jul in 2019 and 13 Aug in 2020. No statistically significant differences were observed in the daily values between the treatments during the experiment. The values after harvest represent GP(1000) of the CC which remained low, being 0.82 ± 0.56 g CO2 m−2 h−1 on average.
Predicted gross photosynthesis at PAR level 1000 µmol photon m−2 s−1 (GP1000) for individual plots and measurement days during the growing seasons (n = 292) in the conventional tillage (CT) plots (red dots) and no-till plots (NT; blue dots). Solid lines represent the mean value of the treatments and annual modelling periods (May–May) are marked with light grey or white background. Sowings and harvestings are marked with grey vertical lines and ploughing of CT plots with black vertical lines
The hourly time series of predicted GP followed well the pattern observed for LAI and PAR (Fig. 4b, c, e). There were no significant differences in the modelled GP between the treatments except in the autumns of the years under study when there were still weak swards of the CC in the NT plots while CT plots were tilled (Fig. 4e). However, this was the only time during the whole monitoring period with a notable effect of the CC on GP. Its photosynthesis can be estimated to be approximately only 2.6% of the total GP.
Daily mean of soil temperature and precipitation a photosynthetically active radiation (PAR) b leaf area index (LAI) c which were used as background values in modelling, and model predicted ecosystem respiration d gross photosynthesis e and net ecosystem exchange f of the conventionally tilled (CT) and no-till (NT) plots. Annual modelling periods (May–May) are marked with light grey and white background. Sowings and harvestings are marked with grey vertical lines and ploughing of CT plots with black vertical lines. LAI values > 3 were set to 3 as they did not affect GP due to saturation of the reflectance at modelling. CC represents the LAI of the cover crop
The estimated annual GP was on average 7.3 ± 0.3 Mg CO2–C ha−1 yr−1 (26.8 ± 1.1 Mg CO2-eq ha−1 yr−1) over all years and treatments. Results based on a linear mixed model are referred to as estimated annual averages and in these cases standard errors are shown instead of standard deviations. The annual GP was significantly dependent on the year and there was also a significant interaction of year and management (Table 5). Year 2020 featured the lowest annual GP values compared to other years, and GP was significantly lower in NT in 2019 compared to CT (p = 0.018) but the opposite in 2020 (p = 0.025) (Table 4). CC did not have a statistically significant effect on GP but CC plots had on average 0.48 Mg CO2–C ha−1 yr−1 higher GP than plots without CC.
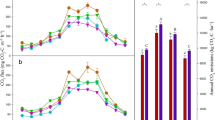
ER was dependent on temperature and LAI, and the highest peaks occurred in the beginning of August and the lowest in February in all years (Fig. 5). No differences were observed in ER between CT and NT on individual measurement days in 2018 but there were occasions of higher ER in CT compared to NT in Jun 2019 and in Mar, Apr, May and Dec 2020 whereas it was lower in CT once in Jul 2019. The annual variation in the measured and predicted (Fig. 4d) ER values followed a similar pattern.
Ecosystem respiration in the individual opaque chambers (dots) and their mean value (line) in conventional tillage (CT) and no-till (NT). Annual periods (May - Apr) are marked with light grey and white background. Sowings and harvests are marked with grey vertical lines and ploughing of CT plots with black vertical lines. Asterisks denote statistically significant differences between treatments (one-way ANOVA, p<0.05), and the colour indicates the treatment with the higher value
On average, the estimated annual ER was 13.5 ± 0.3 Mg CO2–C ha−1 yr−1 (49.7 ± 1.1 Mg CO2-eq ha−1 yr−1) over all years and both management methods. Statistically significant differences between years or tillage management methods were not found (Table 5). Only CC had a statistically significant effect on ER; CC plots had 0.9 Mg CO2–C ha−1 yr−1 higher ER than plots without CC (results not shown).
Temporal variation in NEE followed mostly that of ER (Fig. 4d, f). Daily average NEE was positive during most of the time in this experiment, i.e. more carbon was released than bound in the field ecosystem. The NEE was negative only on 19 days in 2018, 23 days in 2019 and 5 days in 2020.
The estimated annual NEE was 6.2 ± 0.4 Mg CO2–C ha−1 yr−1 (22.9 ± 1.6 Mg CO2-eq ha−1 yr−1) across all years and managements. NEE did vary between years, being lower in 2018 compared to 2020 (p = 0.013). No other significant effects were found but NEE tended to be higher in CT compared to NT (Table 4).
The crop yield carbon was higher in the barley year 2019 compared to the oat years 2018 and 2020, and there was no difference in the yield carbon of years 2018 and 2020 (Tables 5 and 4). Tillage managements or CC did not have any effect on the measured yields.
On average, the estimated net ecosystem carbon balance was 7.9 ± 0.4 Mg CO2-C ha−1 yr−1 (28.8 ± 1.6 Mg CO2-eq ha−1 yr−1 for the whole period under study and all management options. The results on carbon balance followed closely those of NEE as they are calculated as the sum of the NEE and yield. Net ecosystem carbon balance was significantly lower in 2018 compared to 2020 (p = 0.008) but no other statistically significant differences were found (Table 5). The proportion of biomass carbon removed from the yield was 23% on average.
Measurements of respiration on bare soil with the small soil respiration chamber (Fig. 6) showed more variability than those done with the larger opaque chamber (Fig. 4). This method also resulted in generally higher emissions compared to the opaque chambers during the winter season. For example, in the winter period from Oct 2019 to May 2020, average CO2 flux was 0.22 g CO2 m−2 h−1 with the opaque chamber method in both treatments (n = 88 in CT and NT) while the soil respiration chambers showed an average of 0.61 and 0.26 g CO2 m−2 h−1 in CT and NT (n = 72), respectively. CT plots had higher respiration rates compared to NT plots particularly in early summers, and in autumns after ploughing of the years under study. Only in the early autumn of 2019 had the NT plots higher respiration rates than the CT plots. Soil respiration was significantly related to soil temperature, but model correlation was poor due to high variation in the measured data, especially in CT (Fig. 7; Table 6). Between sowing and harvesting, the total model predicted soil respiration was (mean ± standard error, Mg CO2–C ha−1) 7.3 ± 0.6 and 6.3 ± 0.6 in CT and 8.1 ± 0.4 and 6.5 ± 0.4 in NT in 2019 and 2020, respectively. Correspondingly, ER (mean ± standard error, Mg CO2–C ha−1) was 10.8 ± 0.4 and 9.8 ± 0.4 in CT, and 10.4 ± 0.2 and 10.2 ± 0.3 in NT during the same periods. On average, the proportion of soil respiration of ecosystem respiration was 74% in 2019 and 65% in 2020.
Respiration of bare soil in conventional tillage (CT) and no-till (NT). Annual periods (May-Apr) are marked with light grey and white background. Sowings and harvestings are marked with grey vertical lines and ploughing of CT plots with black vertical lines. Asterisks denote statistically significant differences between treatments (one-way ANOVA, p < 0.05), and the colour indicates the treatment with the higher value
Bare soil flux measurements in relation to soil temperature from conventional tillage (CT) plots and no-tillage (NT) plots. In 2019, soil temperature was measured at the depth of 10 cm and 5 cm in 2020. Solid lines represent fitted soil respiration model (equation x) for which details are explained in Table x. n = 140 and 167 for CT and 125 and 163 for NT in 2019 and 2020, respectively. Dashed lines denote the 95% confidence intervals
There was a high CO2 peak in soil respiration immediately after ploughing, 14.6 ± 4.2 g CO2 m−2 h−1 on average, but half an hour later the respiration rate was only 45% of that (Fig. 8). With time since ploughing, the soil respiration level decreased further, being about 27%, 20%, 10% of the peak value at 1.5, 3.5 and 23 h after ploughing, respectively. The CO2 flux settled down to approximately the same level as before ploughing in three days (0.6 g CO2 m−2 h−1, about 5% of the peak). Cumulative soil carbon loss during the first three days after ploughing was 0.34 ± 0.7 Mg CO2–C ha−1 which was 0.22 ± 0.06 Mg CO2–C ha−1 more in comparison to the value based on the respiration level before ploughing. After ploughing the CO2 fluxes remained higher in the CT plots compared to the NT plots until 29 January next year. In relation to the total annual ER, the proportion of C loss caused by ploughing was 2% on average, ranging from 1 to 4% within the individual CT plots.
N2O flux
During the three years of the experiment, N2O flux was 0.15 mg N2O m−2 h−1 on average and it varied between 0.008 and 1.3 mg N2O m−2 h−1 on individual measurement days. The highest N2O rates occurred in the spring during the frost melt in 2019 and 2021, whereas in 2020 the highest rates occurred in July (Fig. 9). In the spring of 2021, the peaks likely caused by frost melting appeared in both management methods but they were two weeks earlier in CT than in NT. The emission levels remained at a relatively high rate for about one month after sowing and fertilisation but lowered towards the late summer. Statistically significant differences in the daily N2O levels between treatments were rare before summer 2020. During the first two years, NT had higher emissions compared to CT on several occasions but from the summer of 2020 on, it was more common to have lower emission levels from NT.
N2O flux in individual opaque chambers (dots) and their mean value (line) in conventional tillage (CT) and no-till (NT). Annual periods (May–Apr) are marked with light grey and white background. Sowings and harvestings are marked with grey vertical lines and ploughing of CT plots with black vertical lines. Asterisks denote statistically significant differences between treatments (one-way ANOVA, p < 0.05), and the colour indicates the treatment with the higher value
The estimated annual N2O emission rate was on average 7.9 ± 0.7 kg N2O–N ha−1 yr−1 (3.4 ± 0.3 Mg CO2-eq ha−1 yr−1) for the whole period under study and all the management options employed. The annual emission level of N2O varied significantly by year (Table 5). In 2019 when barley was the main crop, N2O emissions were generally lower than in other years (p < 0.002). There was also a significant interaction of year and management showing lower N2O emissions in CT management in 2020 compared to other years (p < 0.002), but there were no differences between the years in NT. There was also a significant difference between the tillage managements in 2020 when NT had lower N2O emissions compared to CT (p = 0.044) (Table 4). CC did not have a significant effect on N2O (p = 0.15) but plots with CC tended to have a lower N2O rate (7.2 ± 0.7 kg N2O–N ha−1 yr−1) compared to plots without CC (8.7 ± 0.7 kg N2O–N ha−1 yr−1).
CH4 flux
CH4 fluxes varied from a slight sink to a moderate source during the whole monitoring period (Fig. 10). The mean CH4 flux of individual measurement days was − 0.007 mg CH4 m−2 h−1 on average ranging from − 0.036 to 0.072 during the experiment. There were no distinct peaks in the flux rates except a minor increase in the emissions in the late summer 2020. The fluxes were higher (higher emissions or lower sink) in NT compared to CT on four measurement days: in November 2019 and in April 2019, 2020 and 2021.
Methane flux in individual opaque chambers (dots) and their mean value (line) in conventional tillage (CT) and no-till (NT). Annual periods (May–Apr) are marked with light grey and white background. Sowings and harvestings are marked with grey vertical lines and ploughing of CT plots with black vertical lines. Significant differences (one-way ANOVA, p < 0.05) are marked with asterisks which is coloured according to the higher value of treatments
At the annual level, the experimental field was a slight CH4 sink for all management methods and years under study. On average, the estimated CH4 emission rate was − 0.41 ± 0.11 kg CH4–C ha−1 yr−1 (−0.015 ± 0.004 Mg CO2-eq ha−1 yr−1) over all years and managements. Annual CH4 emissions differed by year (Table 5) with a higher sink in 2018 compared to 2019 (p = 0.079) and 2020 (p = 0.025).
Annual GHG balance
The estimated total GHG balance as CO2 equivalents of the three GHGs (GWP-100) was 26.3 ± 1.9 Mg CO2-eq ha−1 yr−1 over all the years and management methods. On average, the proportion of CO2 of the climate warming effect was 85%, whereas it was 15% for N2O and 0.07% for CH4. Tillage treatments differed significantly with respect to their total GHG balance (Table 5) with generally lower emissions from NT compared to CT (Table 4). Annually, a significant difference between CT and NT was found only in 2018 (p = 0.020).
Discussion
With the average NEE of 6.2 ± 2.8 Mg CO2–C ha −1 yr−1 during the experiment, our estimate of the carbon balance is in line with other studies from northern European cultivated peatlands. Maljanen et al. (2010) reported an average NEE of 4.8 ± 3.2 Mg CO2–C ha−1 yr−1 from cereal cultivation on peatlands in Nordic countries. Likewise, the NEE has been estimated to be 2.1 (Lohila et al. 2004), 4.1 (Maljanen et al. 2001) and 8.2 Mg CO2–C ha−1 yr−1 (Maljanen et al. 2004) for Finnish barley sites. The results by Elsgaard et al. (2012) showed NEE of 8.6 ± 2.0 Mg CO2–C ha−1 yr−1 for cultivated peatlands in Denmark (13 ± 4.5 Mg CO2–C ha−1 yr−1 for barley). On the global scale, Tan et al. (2020) reported average of 5.3 ± 2.2 Mg CO2–C ha−1 yr−1 for cultivated peat soils.
Even though the field has been cultivated for at least 160 years, and the surface layer is highly decomposed (Table 1), the emissions still continue at a rate typical for cultivated peat soils. It has been shown that the CO2 emissions of cultivated peat soils do not necessarily decrease with lowering carbon content (Norberg et al. 2018; Säurich et al. 2019), and CO2 emissions from soils that have a lower carbon content than “real” peatlands can be as high as those from peat soils (Leiber-Sauheitl et al. 2014; Tiemeyer et al. 2016). The carbon content of our site is already relatively low, but the emissions might continue at a similar level as long as the majority of the two-metre peat layer is consumed. Our results for the net ecosystem carbon balance (mean 8.0 ± 2.4 Mg C ha−1 yr−1) fall within the confidence interval of the default IPCC emission factor for the boreal region, 6.5–9.4 Mg CO2–C ha−1 yr−1 (IPCC 2014). Although Finland is a relatively cold country, the aggregated IPCC emission factor for temperate and boreal regions thus seems to be suitable for reporting CO2 emissions in northern conditions.
The mean N2O emissions during our experiment (7.9 kg N2O–N ha−1 yr−1) were at the lower end of the reported range found in the literature. In the review study by Maljanen et al. (2010) the average N2O emissions from cereal cultivated peatlands in Nordic countries was 10 ± 3.2 kg N2O–N ha−1 yr−1. Values from Finnish barley sites were 8.3 (Maljanen et al. 2003c), 8.3 (Maljanen et al. 2004), 15 and 13 (Regina et al. 2004) kg N2O–N ha−1 yr−1. The observed variation is, however, extremely high: e.g. Kandel et al. (2018) found emission rate of 0.95 ± 0.76 kg N2O−N ha−1 yr−1 (oats-spring barley rotation) in Denmark while Taft et al. (2017) reported 17 ± 1.7 kg N2O–N ha−1 yr−1 for cultivated peat soils in the UK. Our mean value is just below the 95% confidence interval of the IPCC default emission factor for N2O emissions for boreal and temperate regions, 8.2–18 kg N2O–N ha−1 yr−1 (IPCC 2014).
The N2O flux showed a high peak after the frost melted in spring 2019 and 2021. This is typical for melting soils, especially after a winter with frequent freeze-thaw episodes (Kaiser et al. 1998; Regina et al. 2004). High N2O fluxes in the spring occur when the diffusion barrier of ice is removed, physical structures break and the substrate level increases, enhancing the microbial activity (Wagner-Riddle et al. 2017). In spring 2020, no distinct peaks were observed, likely due to a warm winter with no freeze-thaw cycles. CT had a higher and earlier peak of N2O compared to NT most likely due to faster melting of the ploughed soil as found also in an earlier study (Regina and Alakukku 2010). Frost melted later in the denser NT soil which can be clearly seen in the flux in the spring 2021. The peaks in July 2020 and July 2018 were most likely due to the first heavy rain after fertilisation enhancing denitrification.
The soil was well drained and mainly acted as a sink of CH4. The observed annual mean CH4 sink of − 0.4 kg CH4–C ha−1 yr−1 compares well with most previously studied Finnish barley sites that have also mainly been small CH4 sinks; the reported values have been − 1.8 (Maljanen et al. 2003b), -0.75 (Maljanen et al. 2004), − 0.23 and 1.8 (Regina et al. 2007) kg CH4–C ha−1 yr−1. Our observation is also in line with the findings of Kandel et al. (2018) who observed − 1.05 ± 2.2 kg CH4−C ha−1 yr−1 CH4 sink for oat-barley rotation on a Danish peatland. Especially poorly drained cultivated peat soils can turn from sinks to be sources of CH4. Taft et al. (2017) reported CH4 emissions of 1.2 ± 0.6 kg CH4–C ha−1 yr−1 and Maljanen et al. (2010) mean of 1.57 (range from − 1.8 to 11.7) kg CH4–C ha−1 yr−1 for Nordic countries. No specific peaks clearly related to environmental variables occurred except an occasion of CH4 emissions after a heavy rain following a dry period in the summer 2020.
As soil respiration was 60–70% of the total respiration, it is the most significant component of the carbon balance in this field. It is thus important to find ways to reduce carbon losses via this pathway, especially in carbon-rich soils where the ordinary carbon input cannot counteract losses of peat decomposition (Freeman et al. 2022). With this respect, NT is a promising practice since it has the potential to reduce soil respiration due to less soil disturbance (Chatskikh et al. 2008; Akbolat et al. 2009). Like in the study of Elder and Lal (2008a), there was a tendency to lower CO2 emissions in NT compared to CT at our site. Our observations on increased CO2 emissions immediately after ploughing are in line with Reicosky et al. (1997) and Vinten et al. (2002). Reicosky et al. (1997) reported CO2 emissions of 0.32 Mg CO2–C ha−1 within 24 h from the ploughing of carbon rich black soil which is very close to our three-day result 0.34 ± 0.7 Mg CO2–C ha−1. Vinten et al. (2002) reported a high peak after ploughing but the emissions settled to the same level with NT within two days resulting in no difference in long-term CO2 emissions from clay loam soil. Although the CO2 emissions were momentarily high right after ploughing, they had a minor effect on the cumulative annual emissions. Furthermore, there were no consistent differences in the annual CO2 emissions between the treatments throughout the years.
N2O emissions were 31% lower in the NT treatment compared to CT in 2020 mainly due to higher emission rates in CT during the summer period which may be due to higher nitrate concentration in the more disturbed CT soil. We do not have the data from 2020 but the mineral nitrogen results from summer 2019 indicated clearly higher nitrate levels in the CT plots compared to NT plots (results not shown). Our results are similar with those of (Elder and Lal 2008a) who found a significant reduction (63%) of N2O emissions in NT management on organic soil, but in their case this was likely due to increased topsoil density in NT. Denser soil in NT promotes more complete denitrification towards higher proportion of N2 in the end products (Liu et al. 2007), but at the site of the present study we did not observe differences either in soil moisture (results not shown) nor in density or porosity. However, increased N2O emissions in NT have also been commonly reported (Oorts et al. 2007; Regina and Alakukku 2010; Sheehy et al. 2013). Ball (2013) concluded that the surface layer is the main source of N2O emissions and a poor soil structure with restricted air permeability and aeration may result in high denitrification rate. These results are mainly based on studies on mineral soils, and it is not clear if the same applies in peat soils in which soil structure does not as strongly determine soil functions. The contrasting observations may also partly result from the differences in study duration. A meta-analysis of Van Kessel et al. (2013) showed that there was a significant decrease of N2O emissions in long-term (> 10 year.) and increase in short-term NT management under dry climate conditions. Our experiment was relatively short, and longer NT management would confirm how much NT eventually compacts the soil, and what kind of implications it has on the trace gas fluxes.
Cover crops are used to reduce the amount of free nutrients in the soil or to provide nitrogen fixation or carbon input to agricultural soils (Poeplau and Don 2015). There is limited data on the effects of cover crop in peat soils, but the available data suggests that while cover crop can decrease N2O emissions, its incorporation can prime peat oxidation and CO2 emissions (Wen et al. 2019). We were unable to provide solid information on the potential impacts of a typical cover crop sward on the soil processes in this experiment as the cover crop yielded very little biomass and consequently had only minor effect on ecosystem respiration. Our CC yields were clearly below the mean Italian ryegrass biomass of 2.2 Mg dry matter ha−1 observed in boreal climatic conditions (Känkänen 2019) or the < 1 Mg ha−1 biomass estimated necessary for carbon sequestration (Blanco-Canqui et al. 2020). Although there is a need to counteract the short growing season with additional vegetation cover, the harsh climate of Finland has been found to limit the success of CCs. In a questionnaire to 1100 farmers less than half of the respondents reported an extensive soil cover by CC (Peltonen-Sainio et al. 2022).
Modelling peatland dynamics may require specialized approaches to accurately capture peatland-specific processes (Mozafari et al. 2023). Part of the uncertainty in the GHG results arises from the simplicity of the models as some parameters were general values from published studies. For example, soil respiration was modelled only based on soil temperature, although it can be affected also by such factors as changes in microbial community composition or activity (Yang et al. 2022) and soil moisture (Smith et al. 2018). In particular, the soil respiration chamber measurements showed higher fluxes from CT compared to NT after ploughing in the growing seasons of 2019 and 2020, and the higher fluxes continued until the frost. It seems, however, that the model for soil respiration was not capable of showing such a difference between the treatments. One reason for the difference in soil respiration measurements may be due to the stronger effect of air mixing in CT with a more porous soil surface compared to NT. Air mixing has been found to be a major source of error (Pumpanen et al. 2004). Estimating vegetation cover using measured LAI is also problematic, as it reflects weakly the amount of active chlorophyll during cereal ripening (Gregersen et al. 2013; Delegido et al. 2015). The interpolation used in the models from the peak value of LAI to the end of August is a clear simplification, but it is in good agreement with the models and the measured GP values. Furthermore, LAI was at the same level in 2019 as in 2020 but lower in 2018 although there was no clear difference in yields or GP. This may be related to the varying performance of the measuring device. The differences are not highly relevant in the modelling, as each year is dealt with separately, but they may cause extra variation in the results and explain the high error. The measurement results of PAR values also contain uncertainties due to possible changes in cloudiness, fogging or dirt on the plexiglass and other shading, although seemingly erroneous observations were removed in the data filtering. The plexiglass surfaces were kept as clean as possible, and fogging was kept low with a short measurement time. Averaged air temperature in gap filling of soil temperature may cause some error, but the filled gaps were not long, and the error was mostly diurnal with low risk of significant errors in annual balances. With N2O and CH4 measurements every two weeks, there is a high risk if missing short-term peaks for example due to freeze-thaw cycles (Lammirato et al. 2021). Also, if the measurements hit peaks, the emissions may be overestimated due to interpolation of the gaps in the data particularly during times with infrequent measurements. However, even with these uncertainties it is plausible to assume the climatic benefits of NT in boreal peat soils are moderate and other, more effective management options for GHG mitigation should be prioritised.
Conclusions
As there was no difference in the grain yields between the cultivation methods, NT can be considered a feasible practice in peat soils from the agronomic viewpoint. As hypothesised, ploughing caused a high but short CO2 emission peak in CT but not in NT without such soil surface disturbance. However, as the majority of carbon loss occurred during the warm period of the year, the effect of NT on the annual carbon loss was of minor significance. Based on previous studies on Finnish NT field experiments, we hypothesised higher N2O emissions in the NT treatment, but this assumption was not supported by the results of the present study. NT showed a lower 100-year global warming potential and clearer effects might be expected in continuous long-term NT management. In conclusion, NT did not have a clear significant effect on GHG balances over the three-year period, but negative effects were not observed either. Thus, reducing tillage is likely not harmful as regards climate change mitigation, and it might have benefits on general soil health.
Data availability
The datasets generated during the study are available from the corresponding author on request.
References
Akbolat D, Evrendilek F, Coskan A, Ekinci K (2009) Quantifying soil respiration in response to short-term tillage practices: a case study in southern Turkey. Acta Agriculturae Scandinavica Section B — Soil & Plant Science 59:50–56. https://doi.org/10.1080/09064710701833202
Aparicio N, Villegas D, Casadesus J et al (2000) Spectral vegetation indices as nondestructive tools for determining Durum Wheat Yield. AgronJ 92:83–91. https://doi.org/10.2134/agronj2000.92183x
Ball BC (2013) Soil structure and greenhouse gas emissions: a synthesis of 20 years of experimentation. EurJSoil Sci 64:357–373. https://doi.org/10.1111/ejss.12013
Ball BC, Scott A, Parker JP (1999) Field N2O, CO2 and CH4 fluxes in relation to tillage, compaction and soil quality in Scotland. Soil Tillage Res 53:29–39
Blanco-Canqui H, Ruis SJ, Proctor CA et al (2020) Harvesting cover crops for biofuel and livestock production: another ecosystem service? AgronJ 112:2373–2400. https://doi.org/10.1002/agj2.20165
Cai A, Han T, Ren T et al (2022) Declines in soil carbon storage under no tillage can be alleviated in the long run. Geoderma 425:116028. https://doi.org/10.1016/j.geoderma.2022.116028
Chatskikh D, Olesen JE, Hansen EM et al (2008) Effects of reduced tillage on net greenhouse gas fluxes from loamy sand soil under winter crops in Denmark. Agric EcosystEnviron 128:117–126. https://doi.org/10.1016/j.agee.2008.05.010
Delegido J, Verrelst J, Rivera JP et al (2015) Brown and green LAI mapping through spectral indices. Int J Appl Earth Obs Geoinf 35:350–358. https://doi.org/10.1016/j.jag.2014.10.001
EC (2021) Communication from the Commission to the European Parliament and the Council. Sustainable Carbon Cycles. COM(2021) 800 final. Available at https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52021DC0800&qid=1688374710261
Elder JW, Lal R (2008a) Tillage effects on gaseous emissions from an intensively farmed organic soil in North Central Ohio. Soil Tillage Res 98:45–55. https://doi.org/10.1016/j.still.2007.10.003
Elder JW, Lal R (2008b) Tillage effects on physical properties of agricultural organic soils of north central Ohio. Soil Tillage Res 98:208–210. https://doi.org/10.1016/j.still.2007.12.002
Elsgaard L, Görres C-M, Hoffmann CC et al (2012) Net ecosystem exchange of CO2 and carbon balance for eight temperate organic soils under agricultural management. Agric EcosystEnviron 162:52–67. https://doi.org/10.1016/j.agee.2012.09.001
FAO (2020) Drained organic soils 1990–2019. Global, regional and country trends. FAOSTAT Analytical Brief Series No 4, Rome
Forster P, Storelvmo T, Armour K et al (2021) The Earth’s energy budget, climate feedbacks, and climate sensitivity. In Climate Change 2021: the physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Camb Univ Press 923–1054. https://doi.org/10.1017/9781009157896.009
Freeman BWJ, Evans CD, Musarika S et al (2022) Responsible agriculture must adapt to the wetland character of mid-latitude peatlands. Glob Change Biol 28:3795–3811. https://doi.org/10.1111/gcb.16152
Gregersen PL, Culetic A, Boschian L, Krupinska K (2013) Plant senescence and crop productivity. Plant MolBiol 82:603–622. https://doi.org/10.1007/s11103-013-0013-8
Heikkinen J, Ketoja E, Nuutinen V, Regina K Declining trend of carbon in Finnish cropland soils in 1974–2009. Global Change Biology 19:1456–1469
Honkanen H, Turtola E, Lemola R et al (2021) Response of boreal clay soil properties and erosion to ten years of no-till management. Soil Tillage Res 212:105043. https://doi.org/10.1016/j.still.2021.105043
Humpenöder F, Karstens K, Lotze-Campen H et al (2020) Peatland protection and restoration are key for climate change mitigation. Environ Res Lett 15:104093. https://doi.org/10.1088/1748-9326/abae2a
IPCC 2014 (2013) In: Hiraishi T, Krug T, Tanabe K, Srivastava N, Baasansuren J, Fukuda M, Troxler TG (eds) Supplement to the 2006 IPCC guidelines for National Greenhouse Gas inventories: wetlands. IPCC, Switzerland, Published
Jensen LS, Salo T, Palmason F et al (2005) Influence of biochemical quality on C and N mineralisation from a broad variety of plant materials in soil. Plant Soil 273:307–326. https://doi.org/10.1007/s11104-004-8128-y
Jokinen P, Pirinen P, Kaukoranta J-P et al (2021) Tilastoja Suomen ilmastosta ja merestä 1991–2020
Joosten H, Sirin A, Couwenberg J et al (2016) The role of peatlands in climate regulation. Peatland restoration and ecosystem services: science, policy and practice. Cambridge University Press Cambridge, UK, pp 63–76
Kaiser E-A, Kohrs K, Kücke M et al (1998) Nitrous oxide release from arable soil: importance of N-fertilization, crops and temporal variation. Soil Biol Biochem 30:1553–1563. https://doi.org/10.1016/S0038-0717(98)00036-4
Kandel TP, Elsgaard L, Lærke PE (2013) Measurement and modelling of CO2 flux from a drained Fen peatland cultivated with reed canary grass and spring barley. GCB Bioenergy 5:548–561. https://doi.org/10.1111/gcbb.12020
Kandel TP, Lærke PE, Elsgaard L (2018) Annual emissions of CO2, CH4 and N2O from a temperate peat bog: comparison of an undrained and four drained sites under permanent grass and arable crop rotations with cereals and potato. AgricForMeteorol 256–257:470–481. https://doi.org/10.1016/j.agrformet.2018.03.021
Känkänen H (2019) Kerääjäkasvitoimenpiteen laadullinen toteutuminen tiloilla. In: Yli-Viikari A, editor. Maaseutuohjelman (2014–2020) ympäristöarviointi; 2019. Luonnonvara- ja biotalouden tutkimus 63:46–67
Kutzbach L, Schneider J, Sachs T et al (2007) CO2 flux determination by closed-chamber methods can be seriously biased by inappropriate application of linear regression. Biogeosciences 4:1005–1025. https://doi.org/10.5194/bg-4-1005-2007
Lammirato C, Wallman M, Weslien P et al (2021) Measuring frequency and accuracy of annual nitrous oxide emission estimates. Agric For Meteorol 310:108624. https://doi.org/10.1016/j.agrformet.2021.108624
Leiber-Sauheitl K, Fuß R, Voigt C, Freibauer A (2014) High CO2 fluxes from grassland on histic gleysol along soil carbon and drainage gradients. Biogeosciences 11:749–761
Liu XJ, Mosier AR, Halvorson AD et al (2007) Dinitrogen and N2O emissions in arable soils: Effect of tillage, N source and soil moisture. Soil Biol Biochem 39:2362–2370. https://doi.org/10.1016/j.soilbio.2007.04.008
Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. FunctEcol 8:315–323. https://doi.org/10.2307/2389824
Lohila A, Aurela M, Regina K, Laurila T (2003) Soil and total ecosystem respiration in agricultural fields: effect of soil and crop type. Plant Soil 251:303–317. https://doi.org/10.1023/A:1023004205844
Lohila A, Aurela M, Tuovinen J-P, Laurila T (2004) Annual CO2 exchange of a peat field growing spring barley or perennial forage grass. J Geophys Res. https://doi.org/10.1029/2004JD004715
Long SP, Hällgren J-E (1993) Measurement of CO2 assimilation by plants in the field and the laboratory. In: Hall DO, Scurlock JMO, Bolhàr-Nordenkampf HR et al (eds) Photosynthesis and production in a changing environment: a field and laboratory manual. Springer Netherlands, Dordrecht, pp 129–167
Maljanen M, Martikainen PJ, Walden J, Silvola J (2001) CO2 exchange in an organic field growing barley or grass in eastern Finland. Global Change Biol 7:679–692. https://doi.org/10.1111/j.1365-2486.2001.00437.x
Maljanen M, Liikanen A, Silvola J, Martikainen PJ (2003a) Measuring N2O emissions from organic soils by closed chamber or soil/snow N2O gradient methods. Eur J Soil Sci 54:625–631. https://doi.org/10.1046/j.1365-2389.2003.00531.x
Maljanen M, Liikanen A, Silvola J, Martikainen PJ (2003b) Methane fluxes on agricultural and forested boreal organic soils. Soil Use Manage 19:73–79. https://doi.org/10.1111/j.1475-2743.2003.tb00282.x
Maljanen M, Liikanen A, Silvola J, Martikainen PJ (2003c) Nitrous oxide emissions from boreal organic soil under different land-use. Soil BiolBiochem 35:689–700. https://doi.org/10.1016/S0038-0717(03)00085-3
Maljanen M, Komulainen V-M, Hytönen J et al (2004) Carbon dioxide, nitrous oxide and methane dynamics in boreal organic agricultural soils with different soil characteristics. Soil BiolBiochem 36:1801–1808. https://doi.org/10.1016/j.soilbio.2004.05.003
Maljanen M, Sigurdsson BD, Guðmundsson J et al (2010) Greenhouse gas balances of managed peatlands in the nordic countries – present knowledge and gaps. Biogeosciences 7:2711–2738. https://doi.org/10.5194/bg-7-2711-2010
Mozafari B, Bruen M, Donohue S, Renou-Wilson F, O’Loughlin F (2023) Peatland dynamics: a review of process-based models and approaches. Sci Total Environ 877:162890. https://doi.org/10.1016/j.scitotenv.2023.162890
Mutegi JK, Munkholm LJ, Petersen BM, Hansen EM, Petersen SO (2010) Nitrous oxide emissions and controls as influenced by tillage and crop residue management strategy. Soil Biol Biochem 42:1701–1711
Nichols JE, Peteet DM (2019) Rapid expansion of northern peatlands and doubled estimate of carbon storage. Nat Geosci 12:917–921. https://doi.org/10.1038/s41561-019-0454-z
Norberg L, Berglund Ö, Berglund K (2018) Impact of drainage and soil properties on carbon dioxide emissions from intact cores of cultivated peat soils
Ogle SM, Swan A, Paustian K (2012) No-till management impacts on crop productivity, carbon input and soil carbon sequestration. Agric Ecosyst Environ 149:37–49. https://doi.org/10.1016/j.agee.2011.12.010
Ogle SM, Alsaker C, Baldock J et al (2019) Climate and soil characteristics determine where No-Till Management can Store Carbon in soils and mitigate Greenhouse Gas emissions. Sci Rep 9:11665. https://doi.org/10.1038/s41598-019-47861-7
Peltonen-Sainio P, Jauhiainen L, Känkänen H, Joona J, Hydén T, Mattila TJ (2022) Farmers’ experiences of how under-sown clovers, ryegrasses, and Timothy Perform in Northern European Crop Production systems. Agronomy 12:1401. https://doi.org/10.3390/agronomy12061401
Poeplau C, Don A (2015) Carbon sequestration in agricultural soils via cultivation of cover crops – A meta-analysis. Agric Ecosyst Environ 2000:33–41. https://doi.org/10.1016/j.agee.2014.10.024
Pumpanen J, Kolari P, Ilvesniemi H et al (2004) Comparison of different chamber techniques for measuring soil CO2 efflux. Agric for Meteorol 123:159–176. https://doi.org/10.1016/j.agrformet.2003.12.001
Raich JW, Rastetter EB, Melillo JM, et al (1991) Potential net primary productivity in South America: application of a global model. Ecol Appl 1:399–429. https://doi.org/10.2307/1941899
Regina K, Alakukku L (2010) Greenhouse gas fluxes in varying soils types under conventional and no-tillage practices. Soil Tillage Res 109:144–152. https://doi.org/10.1016/j.still.2010.05.009
Regina K, Syväsalo E, Hannukkala A, Esala M (2004) Fluxes of N2O from farmed peat soils in Finland. EurJSoil Sci 55:591–599. https://doi.org/10.1111/j.1365-2389.2004.00622.x
Regina K, Pihlatie M, Esala M, Alakukku L (2007) Methane fluxes on boreal arable soils. Agric EcosystEnviron 119:346–352. https://doi.org/10.1016/j.agee.2006.08.002
Reicosky DC, Dugas WA, Torbert HA (1997) Tillage-induced soil carbon dioxide loss from different cropping systems. Soil Tillage Res 41:105–118. https://doi.org/10.1016/S0167-1987(96)01080-X
Säurich A, Tiemeyer B, Don A et al (2019) Drained organic soils under agriculture — the more degraded the soil the higher the specific basal respiration. Geoderma 355:113911. https://doi.org/10.1016/j.geoderma.2019.113911
Shakoor A, Shahbaz M, Farooq TH et al (2021) A global meta-analysis of greenhouse gases emission and crop yield under no-tillage as compared to conventional tillage. SciTotal Environ 750:142299. https://doi.org/10.1016/j.scitotenv.2020.142299
Smith KA, Ball T, Conen F et al (2018) Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. EurJSoil Sci 69:10–20. https://doi.org/10.1111/ejss.12539
Taft HE, Cross PA, Edwards-Jones G et al (2017) Greenhouse gas emissions from intensively managed peat soils in an arable production system. Agric EcosystEnviron 237:162–172. https://doi.org/10.1016/j.agee.2016.11.015
Tan L, Ge Z, Zhou X et al (2020) Conversion of coastal wetlands, riparian wetlands, and peatlands increases greenhouse gas emissions: a global meta-analysis. Glob Change Biol 26:1638–1653. https://doi.org/10.1111/gcb.14933
Thornley JH, Johnson IR (1990) Plant and crop modelling. Clarendon, Oxford, p 669
Tiemeyer B, Albiac Borraz E, Augustin J et al (2016) High emissions of greenhouse gases from grasslands on peat and other organic soils. Glob Change Biol 22:4134–4149
van Kessel C, Venterea R, Six J et al (2013) Climate, duration, and N placement determine N2O emissions in reduced tillage systems: a meta-analysis. Glob Change Biol 19:33–44. https://doi.org/10.1111/j.1365-2486.2012.02779.x
Vinten AJA, Ball BC, O’Sullivan MF, Henshall JK (2002) The effects of cultivation method, fertilizer input and previous sward type on organic C and N storage and gaseous losses under spring and winter barley following long-term leys. J Agricultural Sci 139:231–243. https://doi.org/10.1017/S0021859602002496
Vuorinen J, Mäkitie O (1955) The method of soil testing in use in Finland. Maatalouskoelaitoksen maatutkimusosasto, Helsinki
Wagner-Riddle C, Congreves KA, Abalos D et al (2017) Globally important nitrous oxide emissions from croplands induced by freeze–thaw cycles. Nat Geosci 10:279–283. https://doi.org/10.1038/ngeo2907
Wen Y, Zang H, Freeman B, Ma Q, Chadwick DR, Jones DL (2019) Rye cover crop incorporation and high watertable mitigate greenhouse gas emissions in cultivated peatland. Land Degrad Dev. https://doi.org/10.1002/ldr.3390
West TO, Marland G (2002) A synthesis of carbon sequestration, carbon emissions, and net carbon flux in agriculture: comparing tillage practices in the United States. Agric EcosystEnviron 91:217–232. https://doi.org/10.1016/S0167-8809(01)00233-X
Yang S, Wu H, Wang Z et al (2022) Linkages between the temperature sensitivity of soil respiration and microbial life strategy are dependent on sampling season. Soil BiolBiochem 172:108758. https://doi.org/10.1016/j.soilbio.2022.108758
Ziegler R (2020) Paludiculture as a critical sustainability innovation mission. Res Policy 49:103979. https://doi.org/10.1016/j.respol.2020.103979
Acknowledgements
Assistance of Merja Myllys in the experiment setup and Luke technical staff in the field and laboratory work is gratefully acknowledged. The concept for this paper was developed at the workshop titled “Peatlands for climate change mitigation in agriculture” that took place in Aarhus, Denmark, on 4–5 October 2022, and which was sponsored by the Organisation for Economic Co-operation and Development (OECD) Co-operative Research Programme: Sustainable Agricultural and Food Systems. The opinions expressed and arguments employed in this publication are the sole responsibility of the authors and do not necessarily reflect those of the OECD or of the governments of its Member countries.
Funding
Open access funding provided by Natural Resources Institute Finland. The work was part of the project SOMPA (Novel soil management practices – key for sustainable bioeconomy and climate change mitigation), funded by the Strategic Research Council at the Research Council of Finland, grant no. 312912. The work of HH was also funded by Maa- ja vesitekniikan tuki ry.
Author information
Authors and Affiliations
Contributions
KL and HK designed the study, HH and HK maintained the experimental site, performed sampling and analysed the data, HH and JK performed statistical analyses, HH made the figures and tables and the first version of the manuscript. All authors commented the manuscript versions and read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Klaus Butterbach-Bahl.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Honkanen, H., Kekkonen, H., Heikkinen, J. et al. Minor effects of no-till treatment on GHG emissions of boreal cultivated peat soil. Biogeochemistry (2023). https://doi.org/10.1007/s10533-023-01097-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10533-023-01097-w