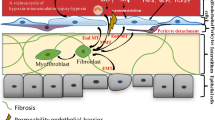
Abstract
Vascular endothelial dysfunction is a major risk factor in the development of renal diseases. Recent studies pointed out a major interest for the inter-endothelial junction protein CD146, as its expression is modulated during renal injury. Indeed, some complex mechanisms involving this adhesion molecule and its multiple ligands are observed in a large number of renal diseases in fundamental or clinical research. The purpose of this review is to summarize the most recent literature on the role of CD146 in renal pathophysiology, from experimental nephropathy to clinical trials.




Similar content being viewed by others
Availability of data and material
No data availability.
References
Marcu R, Choi YJ, Xue J et al (2018) Human organ-specific endothelial cell heterogeneity. IScience 4
Herzlinger D, Hurtado R (2014) Patterning the renal vascular bed. Semin Cell Dev Biol 36
Wong BW, Marsch E, Treps L, Baes M, Carmeliet P (2017) Endothelial cell metabolism in health and disease: impact of hypoxia. EMBO J 36
Jourde-Chiche N, Fakhouri F, Dou L et al (2019) Endothelium structure and function in kidney health and disease. Nat Rev Nephrol 15
Makris K, Spanou L (2016) Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev 37
Levey AS, Eckardt KU, Tsukamoto Y et al (2005) Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67
Geevarghese A, Herman IM (2014) Pericyte-endothelial crosstalk: Implications and opportunities for advanced cellular therapies. J Transl Res 163
Verma SK, Molitoris BA (2015) Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol 35
Goligorsky MS (2015) Pathogenesis of endothelial cell dysfunction in chronic kidney disease: a retrospective and what the future may hold. Kidney Res Clin Pract 34
Kalucka J, de Rooij LPMH, Goveia J et al (2020) Single-cell transcriptome atlas of murine endothelial cells. Cell 180
Molema G, Aird WC (2012) Vascular heterogeneity in the kidney. Semin Nephrol 32(2)
Dumas SJ, Meta E, Borri M et al (2020) Single-cell RNA sequencing reveals renal endothelium heterogeneity and metabolic adaptation to water deprivation. J Am Soc Nephrol 31
Rosivall L, Peti-Peterdi J (2006) Heterogeneity of the afferent arteriole--correlations between morphology and function. Nephrol Dial Transplant 21
Guerci P, Ergin B, Ince C (2017) The macro- and microcirculation of the kidney. Best Pract Res Clin Anaesthesiol 31
Wang K, Kestenbaum B (2018) Proximal tubular secretory clearance: a neglected partner of kidney function. Clin J Am Soc Nephrol 13
Bobulescu IA, Moe OW (2006) Na+/H+ Exchangers in renal regulation of acid-base balance. Semin Nephrol 26(5)
Stanton BA, Giebisch GH (1982) Potassium transport by the renal distal tubule: effects of potassium loading. Am J Physiol Renal Fluid Electrolyte Physiol 12
Eisner C, Faulhaber-Walter R, Wang Y et al (2010) Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int 77
Berkhin EB, Humphreys MH (2001) Regulation of renal tubular secretion of organic compounds. Kidney Int 59
Pannabecker TL, Layton AT (2014) Targeted delivery of solutes and oxygen in the renal medulla: role of microvessel architecture. Am J Physiol Renal Physiol 307
Pober JS, Sessa WC (2007) Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 7
Sandoo A, Veldhuijzen van Zanten JJCS, Metsios GS, Carroll D, Kitas GD (2015) The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J 4(1)
Neubauer K, Zieger B (2022) Endothelial cells and coagulation. Cell Tissue Res 387
Jin J, Fang F, Gao W, Chen H, Wen J, Wen X et al (2021) The structure and function of the glycocalyx and its connection with blood-brain barrier. Front Cell Neurosci 15
Roumenina LT, Rayes J, Frimat M, Fremeaux-Bacchi V (2016) Endothelial cells: source, barrier, and target of defensive mediators. Immunol Rev 274
Rezaie AR (2014) Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb Haemost 112
Nomura K, Liu N, Nagai K et al (2007) Roles of coagulation pathway and factor Xa in rat mesangioproliferative glomerulonephritis. Lab Invest 87
Moussa L, Apostolopoulos J, Davenport P, Tchongue J, Tipping PG (2007) Protease-activated receptor-2 augments experimental crescentic glomerulonephritis. Am J Pathol 171
Chung H, Ramachandran R, Hollenberg MD, Muruve DA (2013) Proteinase-activated receptor-2 transactivation of epidermal growth factor receptor and transforming growth factor-β receptor signaling pathways contributes to renal fibrosis. J Biol Chem 288
Caveda L, Corada M, Padura IM et al (1994) Structural characteristics and functional role of endothelial cell to cell junctions. Endothelium 2
Dejana E, Del Maschio A (1995) Molecular organization and functional regulation of cell to cell junctions in the endothelium. Thromb Haemost
Lum H, Malik AB (1994) Regulation of vascular endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 267
Vestweber D (2000) Molecular mechanisms that control endothelial cell contacts. J Pathol 190
Bardin N, George F, Mutin M et al (1996) S-Endo 1, a pan-endothelial monoclonal antibody recognizing a novel human endothelial antigen. Tissue Antigens 48
Johnson JP, Rothbcher U, Sers C (1993) The progression associated antigen MUC18: a unique member of the immunoglobulin supergene family. Melanoma Res 3
Wang Z, Yan X (2013) CD146, a multi-functional molecule beyond adhesion. Cancer Lett 330
Dye DE, Karlen S, Rohrbach B et al (2009) hShroom1 links a membrane bound protein to the actin cytoskeleton. Cell Mol Life Sci 66
Bu P, Zhuang J, Feng J, Yang D, Shen X, Yan X (2007) Visualization of CD146 dimerization and its regulation in living cells. Biochim Biophys Acta Mol Cell Res 1773
Stowell SR, Cho M, Feasley CL et al (2009) Ligand reduces galectin-1 sensitivity to oxidative inactivation by enhancing dimer formation. J Biol Chem 284
Leroyer AS, Blin MG, Bachelier R, Bardin N, Blot-Chabaud M, Dignat-George F (2019) CD146 (cluster of differentiation 146): an adhesion molecule involved in vessel homeostasis. Arterioscler Thromb Vasc Biol
Bardin N, Moal V, Anfosso F et al (2003) Soluble CD146, a novel endothelial marker, is increased in physiopathological settings linked to endothelial junctional alteration. Thromb Haemost 90
Bardin N, Francès V, Combes V, Sampol J, Dignat-George F (1998) CD146: biosynthesis and production of a soluble form in human cultured endothelial cells. FEBS Lett 421
Bardin N, Blot-Chabaud M, Despoix N et al (2009) CD146 and its soluble form regulate monocyte transendothelial migration. Arterioscler Thromb Vasc Biol 29
Anfosso F, Bardin N, Francès V et al (1998) Activation of human endothelial cells via S-Endo-1 antigen (CD146) stimulates the tyrosine phosphorylation of focal adhesion kinase p125 (FAK). J Biol Chem 273
Wang D, Duan H, Feng J et al (2020) Soluble CD146, a cerebrospinal fluid marker for neuroinflammation, promotes blood-brain barrier dysfunction. Theranostics 10
Kaspi E, Heim X, Granel B et al (2017) Identification of CD146 as a novel molecular actor involved in systemic sclerosis. J Allergy Clin Immunol 140
Vainio O, Dunon D, Aïssi F, Dangy JP, McNagny KM, Imhof BA (1996) HEMCAM, an adhesion molecule expressed by c-kit+ hemopoietic progenitors. J Cell Biol 135
Miner JH, Yurchenco PD (2004) Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol 20
Durbeej M (2010) Laminins. Cell Tissue Res 339
Fujiwara H, Kikkawa Y, Sanzen N, Sekiguchi K (2001) Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through α3β1 and α6β1 integrins. J Biol Chem 276
Petäjäniemi N, Korhonen M, Kortesmaa J et al (2002) Localization of laminin α4-chain in developing and adult human tissues. J Histochem Cytochem 50
Flanagan K, Fitzgerald K, Baker J et al (2012) Laminin-411 is a vascular ligand for MCAM and facilitates TH17 cell entry into the CNS. PLoS One 7
Kawataki T, Yamane T, Naganuma H et al (2007) Laminin isoforms and their integrin receptors in glioma cell migration and invasiveness: evidence for a role of α5-laminin(s) and α3β1 integrin. Exp Cell Res 313
Takkunen M, Ainola M, Vainionpää N et al (2008) Epithelial-mesenchymal transition downregulates laminin α5 chain and upregulates laminin α4 chain in oral squamous carcinoma cells. Histochem Cell Biol 130
Thijssen VL, Rabinovich GA, Griffioen AW (2013) Vascular galectins: regulators of tumor progression and targets for cancer therapy. Cytokine Growth Factor Rev 24
Barondes SH, Castronovo V, Cooper DNW et al (1994) Galectins: a family of animal β-galactoside-binding lectins. Cell 76
Stowell SR, Arthur CM, Mehta P et al (2008) Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem 283
Croci DO, Cerliani JP, Dalotto-Moreno T et al (2014) Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell 156
Burns JS, Kristiansen M, Kristensen LP et al (2011) Decellularized matrix from tumorigenic human mesenchymal stem cells promotes neovascularization with galectin-1 dependent endothelial interaction. PLoS One 6
Jouve N, Despoix N, Espeli M et al (2013) The involvement of CD146 and its novel ligand galectin-1 in apoptotic regulation of endothelial cells. J Biol Chem 288
Yazawa EM, Geddes-Sweeney JE, Cedeno-Laurent F et al (2015) Melanoma cell galectin-1 ligands functionally correlate with malignant potential. J Invest Dermatol 135
Kaltner H, Gabius HJ (2012) A toolbox of lectins for translating the sugar code: the galectin network in phylogenesis and tumors. Histol Histopathol 27
Smetana K, André S, Kaltner H, Kopitz J, Gabius HJ (2013) Context-dependent multifunctionality of galectin-1: a challenge for defining the lectin as therapeutic target. Expert Opin Ther Targets 17
Colomb F, Wang W, Simpson D et al (2017) Galectin-3 interacts with the cell-surface glycoprotein CD146 (MCAM, MUC18) and induces secretion of metastasispromoting cytokines from vascular endothelial cells. J Biol Chem 292
Zhang Z, Zheng Y, Wang H, Zhou Y, Tai G (2018) CD146 interacts with galectin-3 to mediate endothelial cell migration. FEBS Lett 592
Zhang Z, Miller MC, Xu X et al (2019) NMR-based insight into galectin-3 binding to endothelial cell adhesion molecule CD146: evidence for noncanonical interactions with the lectin’s CRD β-sandwich F-face. Glycobiology 29
Donato R, R. Cannon B, Sorci G et al (2012) Functions of S100 proteins. Curr Mol Med 13
Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA (2003) Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol 170
Koch M, Chitayat S, Dattilo BM et al (2010) Structural basis for ligand recognition and activation of RAGE. Structure 18
Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J (2009) The endogenous Toll–like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol 86
Riuzzi F, Sorci G, Donato R (2011) S100B protein regulates myoblast proliferation and differentiation by activating FGFR1 in a bFGF-dependent manner. J Cell Sci 124
Ruma IMW, Putranto EW, Kondo E et al (2016) MCAM, as a novel receptor for S100A8/A9, mediates progression of malignant melanoma through prominent activation of NF-κB and ROS formation upon ligand binding. Clin Exp Metastasis 33
Chen Y, Sumardika IW, Tomonobu N et al (2019) Melanoma cell adhesion molecule is the driving force behind the dissemination of melanoma upon S100A8/A9 binding in the original skin lesion. Cancer Lett 452
Ustach C V., Huang W, Conley-LaComb MK et al (2010) A novel signaling axis of matriptase/PDGF-D/β-PDGFR in human prostate cancer. Cancer Res 70
List K (2009) Matriptase: a culprit in cancer? Future Oncol 5
Tung HH, Lee SL (2017) Physical binding of endothelial MCAM and neural transmembrane protease matriptase - novel cell adhesion in neural stem cell vascular niche. Sci Rep 7
Alexander SP, Fabbro D, Kelly E et al (2015) The concise guide to pharmacology in 2015, the catalytic receptors. Br Pharmacol 172
Witmer AN, Dai J, Weich HA, Vrensen GFJM, Schlingemann RO (2002) Expression of vascular endothelial growth factor receptors 1, 2, and 3 in quiescent endothelia. J Histochem Cytochem 50
Ishida A, Murray J, Saito Y et al (2001) Expression of vascular endothelial growth factor receptors in smooth muscle cells. J Cell Physiol 188
Sweeney MD, Ayyadurai S, Zlokovic B V (2016) Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 19
Armulik A, Genové G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21
Jiang T, Zhuang J, Duan H et al (2012) CD146 is a coreceptor for VEGFR-2 in tumor angiogenesis. Blood 120
Folkman J, Kaipainen A (2004) Genes tell lymphatics to sprout or not. Nat Immunol 5
Karkkainen MJ, Haiko P, Sainio K et al (2004) Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5
Yan H, Zhang C, Wang Z et al (2017) CD146 is required for VEGF-C-induced lymphatic sprouting during lymphangiogenesis. Sci Rep 7
Heldin CH (2013) Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal 11
Onimaru M, Yonemitsu Y, Fujii T et al (2009) VEGF-C regulates lymphangiogenesis and capillary stability by regulation of PDGF-B. Am J Physiol Heart Circ Physiol 297
Shang Q, Zhao L, Wang X, Wang M, Sui SF, Mi LZ (2017) Expression and purification of functional PDGF receptor beta. Biochem Biophys Res Commun 489
Ye Z, Zhang C, Tu T et al (2013) Wnt5a uses CD146 as a receptor to regulate cell motility and convergent extension. Nat Commun 4
Zhang L, Luo Y, Teng X et al (2018) CD146: a potential therapeutic target for systemic sclerosis. Protein Cell 9
Serafini T, Colamarino SA, Leonardo ED et al (1996) Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87
Mehlen P, Guenebeaud C (2010) Netrin-1 and its dependence receptors as original targets for cancer therapy. Curr Opin Oncol 22
Mehlen P, Mazelin L (2003) The dependence receptors DCC and UNC5H as a link between neuronal guidance and survival. Biol Cell 95
Layne K, Ferro A, Passacquale G (2015) Netrin-1 as a novel therapeutic target in cardiovascular disease: to activate or inhibit? Cardiovasc Res 107
Ramesh G, Kwon O, Ahn K (2010) Netrin-1: a novel universal biomarker of human kidney injury. Transplant Proc 42
Aimi F, Georgiopoulou S, Kalus I et al (2015) Endothelial Rictor is crucial for midgestational development and sustained and extensive FGF2-induced neovascularization in the adult. Sci Rep 5
Herriges JC, Verheyden JM, Zhang Z et al (2015) FGF-regulated ETV transcription factors control FGF-SHH feedback loop in lung branching. Dev Cell 35
Fernandes-Freitas I, Owen BM (2020) Metabolic roles of endocrine fibroblast growth factors. Curr Opin Pharmacol 25
Gao Q, Zhang J, Wang X et al (2017) The signalling receptor MCAM coordinates apical-basal polarity and planar cell polarity during morphogenesis. Nat Commun 8
Allen BL, Filla MS, Rapraeger AC (2001) Role of heparan sulfate as a tissue-specific regulator of FGF-4 and FGF receptor recognition. J Cell Biol 155
Stefanska A, Kenyon C, Christian HC et al (2016) Human kidney pericytes produce renin. Kidney Int 90
Kwon O, Miller S, Li N, Khan A, Kadry Z, Uemura T (2010) Bone marrow-derived endothelial progenitor cells and endothelial cells may contribute to endothelial repair in the kidney immediately after ischemia-reperfusion. J Histochem Cytochem 58
Bruno S, Bussolati B, Grange C et al (2009) Isolation and characterization of resident mesenchymal stem cells in human glomeruli. Stem Cells Dev 18
Pippin JW, Kaverina N V., Eng DG et al (2015) Cells of Renin lineage are adult pluripotent progenitors in experimental glomerular disease. Am J Physiol Renal Physiol 309
Abed A, Leroyer AS, Kavvadas P et al (2021) Endothelial-Specific Deletion of CD146 Protects Against Experimental Glomerulonephritis in Mice. Hypertension 77
Li X, Wen J, Dong Y et al (2021) Wnt5a promotes renal tubular inflammation in diabetic nephropathy by binding to CD146 through noncanonical Wnt signaling. Cell Death Dis 12
Wang F, Xing T, Wang N, Liu L (2012) Clinical significance of plasma CD146 and P-selectin in patients with type 2 diabetic nephropathy. Cytokine 57
Briesemeister D, Sommermeyer D, Loddenkemper C et al (2011) Tumor rejection by local interferon gamma induction in established tumors is associated with blood vessel destruction and necrosis. Int J Cancer 128
Roeder SS, Stefanska A, Eng DG et al (2015) Changes in glomerular parietal epithelial cells in mouse kidneys with advanced age. Am J Phys Renal Physiol 309
Stasi A, Franzin R, Divella C, Gesualdo L, Stallone G, Castellano G (2020) Double labeling of PDGFR-β and α-SMA in swine models of acute kidney injury to detect pericyte-to-myofibroblast transdifferentation as early marker of fibrosis. Bio-protocol 10
Zhao Y, Zhao H, Zhang Y et al (2014) Isolation and epithelial co-culture of mouse renal peritubular endothelial cells. BMC Cell Biol 15
Fan Y, Fei Y, Zheng L et al (2018) Expression of endothelial cell injury marker Cd146 correlates with disease severity and predicts the renal outcomes in patients with diabetic nephropathy. Cell Physiolo Biochem Int J Exp Cell Physiol Biochem Pharmacol 48
Ji L, Wu GM, Yang LC, Li L, Xu H (2009) Expression of adhension molecule CD146 in renal tubular epithelial cells and its clinical significance in IgA nephropathy. J Sichuan Univ 40
Feng G, Fang F, Liu C, Zhang F, Huang H, Pu C (2012) CD146 gene expression in clear cell renal cell carcinoma: a potential marker for prediction of early recurrence after nephrectomy. Int Urol Nephrol 44
Wragg J, Finnity JP, Anderson JA et al (2016) MCAM and LAMA4 are highly enriched in tumor blood vessels of renal cell carcinoma and predict patient outcome. Cancer Res 76
Małyszko J, Małyszko JS, Brzosko S, Wołczynski S, Myśliwiec M (2005) Markers of endothelial cell activation/injury: CD146 and thrombomodulin are related to adiponectin in kidney allograft recipients. Am J Nephrol 25
Karbowska A, Boratynska M, Kusztal M, Klinger M (2009) Hyperuricemia is a mediator of endothelial dysfunction and inflammation in renal allograft recipients. Transplant Proc 41
Boratyńska M, Karbowska A, Klinger M (2010) The effect of hyperuricemia on endothelial biomarkers and renal function in kidney allograft recipients. Transplant Proc 42:4074–4077
Liao J, Fu Q, Chen W et al (2020) Plasma soluble CD146 as a potential diagnostic marker of acute rejection in kidney transplantation. Front Med 7
Daniel L, Bardin N, Moal V, Dignat-George F, Berland Y, Figarella-Branger D (2005) Tubular CD146 expression in nephropathies is related to chronic renal failure. Nephron. Exp Nephrol 99
Dursun I, Poyrazoglu HM, Gunduz Z et al (2009) The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease. Nephrology Dial Transplant 24
Prud’homme M, Coutrot M, Michel T et al (2019) Acute kidney injury induces remote cardiac damage and dysfunction through the galectin-3 pathway. JACC Basic Transl Sci 4
Acknowledgements
Figures were made with Servier Medical Art by Servier, which is licensed under a Creative Commons Attribution 3.0 Unported License.
Funding
This work was supported by the French National Research Agency (ANR GalCAM-AKI grant to EG, MBC and CEC, ANR-22-CE14-0045), the French National Institute of Health and Medical Research (Inserm) and Sorbonne University. ER is a doctoral fellow of the French Ministry of National Education (“Ecole Doctorale de Physiologie and Physiopathologie,” ED 394). LB is a fellow of the SFAR (“Société Française d’Anesthésie et de Réanimation”).
Author information
Authors and Affiliations
Contributions
Conceptualization, LB, CEC; writing—original draft preparation, LB, ER, MBC, and CEC; writing—review and editing, LB, ER, EG, FD, MCB, and CEC; supervision, CEC; project administration, CEC; funding acquisition, CEC. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. This study does not involve experiments.
Conflict of interest
The authors declare no competing interests.
Consent for publication
All authors approved the final version of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boutin, L., Roger, E., Gayat, E. et al. The role of CD146 in renal disease: from experimental nephropathy to clinics. J Mol Med 102, 11–21 (2024). https://doi.org/10.1007/s00109-023-02392-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02392-7