Abstract
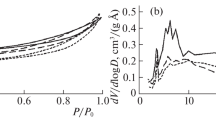
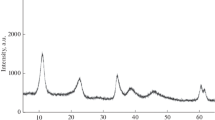
This work studied bulk catalysts for hydrogenation processes of a carbonyl group that have similar compositions and contain copper, zinc, aluminum, and oxygen but were obtained by different methods, such as calcination of a mixture of salts followed by reduction in a hydrogen flow and alkalinization of metal alloys. The effect of the chemical composition and synthesis conditions of such copper-containing catalysts on their surface morphology and textural properties has been studied. The levels of adsorption of hydrogen on the studied catalysts are obtained by combined simultaneous thermal analysis–mass spectrometry. The presence of individual forms of adsorbed hydrogen with different metal–hydrogen bond energies is shown. An explanation for the difference in the adsorption properties relative to hydrogen is provided.




Similar content being viewed by others
REFERENCES
Lapidus, A.L., Ros. Khim. Zh., 2000, vol. 44, no. 1, p. 43.
Prozorov, D.A., Afineevskii, A.V., Knyazev, A.V., et al., Kataliticheskie svoistva i dezaktivatsiya skeletnogo nikelya v reaktsiyakh zhidkofaznoi gidrogenizatsii (Catalytic Properties and Deactivation of Skeletal Nickel in Liquid-Phase Hydrogenation Reactions), Knyazev, A.V., Ed., Kazan: Buk, 2018.
Klyachko, A.L., Kinet. Katal., 1978, vol. 19, no. 5, p. 1218.
Cherdantsev Yu.P., Chernov, I.P., and Tyurin, Yu.I., Metody issledovaniya sistem metall-vodorod (Methods for Investigating Metal-Hydrogen Systems), Tomsk: Tomsk Polytechnic Univ., 2008.
Rumyantsev, R.N., Batanov, A.A., Tsymbalist, I.N., et al., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 2021, vol. 64, no. 10, p. 56.
Prozorov, D.A., Afineevskii, A.V., Smirnov, N.N., et al., Zh. Ross. Khim. O-va, 2017, vol. 61, no. 2, p. 39.
Gizhevskii, B.A., Galakhov, V.R., and Kozlov, E.A., Petrology, 2012, vol. 20, no. 4, p. 317. https://doi.org/10.1134/S0869591112040042
Komarov, Yu.M., Smirnov, N.N., and Il’in, A.P., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 2006, vol. 49, no. 7, p. 48.
Huang, Y.-B., Zhang, J.-L., Zhang, X., et al., Fuel Process. Technol., 2022, vol. 237, p. 107448.
Travin, S.O., Gromov, O.B., Utrobin, D.V., et al., Russ. J. Phys. Chem. B, 2019, vol. 13, no. 6, p. 975. https://doi.org/10.1134/S1990793119060113
Akulova, Yu.P., Problemy termodinamiki poverkhnostnykh yavlenii i adsorbtsii (Problems on Thermodynamics of Surface Phenomena and Adsorption), Ivanovo: Ivanovo State Univ. of Chemistry and Technology, 2009.
Sokol'skii, D.V. and Zakumbaeva, G.D., Adsorbtsiya i kataliz na metallakh VIII gruppy v rastvorakh (Adsorption and Catalysis on Group VIII Metals in Solutions), Alma-Ata: Nauka, 1973.
Mokhov, V.M., Popov, Yu.V., and Bessei, I.B., Izv. Volgogr. Gos. Tekh. Univ., 2013, vol. 10, no. 4, p. 91.
Morandi, F., Longato, B., and Bresadola, S., J. Organomet. Chem., 1982, vol. 239, p. 377.
Rozovskii, A.Ya. and Lin, G.I., Teoreticheskie osnovy protsessa sinteza metanola (Theoretical Foundations for Methanol Synthesis Process), Moscow: Khimiya, 1992.
Chorkendorff, I. and Niemantsverdriet, J.W., Concepts of Modern Catalysis and Kinetics, Wiley-VCH, 2007.
Sazonov, I.V., Izv. Vyssh. Uchebn. Zaved., Neft’ Gaz, 2010, no. 2, p. 117.
ACKNOWLEDGMENTS
The study was performed with the use of the resources of the Center for Collective Use of Scientific Equipment of the Ivanovo State University of Chemistry and Technology.
Funding
The study was financially supported by a grant from the Russian Science Foundation (no. 21-73-10210, https://rscf.ru/project/21-73-10210/). The use of the resources of the Center for Collective Use of Scientific Equipment of the Ivanovo State University of Chemistry and Technology was supported by the Ministry of Science and Higher Education of the Russian Federation (agreement no. 075-15-2021-671).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Prozorov, D.A., Rumyantsev, R.N., Afineevskii, A.V. et al. The Textural and Adsorption Properties of Copper-Containing Catalysts for Carbonyl Group Reduction. Prot Met Phys Chem Surf 59, 854–859 (2023). https://doi.org/10.1134/S2070205123701034
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205123701034