Abstract
Heavy metal (HM) pollution is a threat to ecosystem balance and food security. Cadmium (Cd) is particularly hazardous to humans and accumulates in agricultural soils due to industrial activities and natural sources. Even if Cd adversely affects plant growth and vital functions, few studies have investigated its effects on durum wheat, an important staple crop. Therefore, this study aimed to understand the morphological alteration induced by Cd during the germination phase of durum wheat (Triticum durum L.) seeds to evaluate the tolerance of three cultivars (Karim, Nachit, and Louiza) to Cd stress. The experiment involved germinating wheat seeds in nine concentrations of Cd (0, 0.125, 0.25, 0.375, 0.5, 0.675, 0.75, 0.875, 1 g/L) where germination rate, biomass production, shoot and root length, vigor index, growth inhibition, and tolerance indices were assessed. The different treatments were arranged using a completely randomized design. The results showed that Cd negatively affected wheat germination. The minimum effect of Cd on germination percentage was recorded at 0.125 g/L of Cd for Nachit and Louiza, and at 0.25 g/L of Cd for Karim, while the maximum inhibition was noticed starting at 0.625, 0.875, and 0.625 g/L of Cd for Karim, Nachit, and Louiza, respectively. The negative effect of Cd on shoot and root length was more pronounced during seedling development than during germination. Cd had a greater effect on root length than on shoot length, with Louiza being the most affected cultivar. However, Cd had no significant effect on fresh and dry weight. Our research reveals that the presence of Cd pollution has a substantial impact on the initial growth stages of durum wheat with differential responses of the studied cultivars when exposed to Cd stress. These alterations in seedlings’ performances strongly emphasize the importance of evaluating Cd’s effects on plant development and fitness. This underscores the significance of this aspect not only for ensuring the health of our crops but also for safeguarding food quality in the presence of HM pollution.
Similar content being viewed by others
Introduction
The harmful effects of heavy metal (HM) pollution threaten the balance of the ecosystem (Khan et al. 2015). The presence of HM in soils affects plant growth and development, which could lead to a food security crisis if not properly managed (Ahmad et al. 2022; Zulfiqar et al. 2022b). Cadmium (Cd) is considered the most dangerous heavy metal for humans because of its great ambivalence (Ahmad et al. 2012). Due to urbanization and industrialization, increasing levels of Cd are found in the environment, especially in agricultural soils, as they threaten the food chain (Cheng et al. 2022; Li et al. 2023). The availability of Cd in the environment is due to industrial activities including mining and smelting, application of inorganic fertilizers and irrigation with untreated wastewater, as well as natural sources including mother rock weathering (Nawaz et al. 2021; Zulfiqar et al. 2022b; Li et al. 2023; Sabiha-Javied et al. 2023). Cd is not an essential element; however, plants absorb it when available in soil (Shahid et al. 2017). Cd high toxicity is well pronounced on plants by reducing all vital functions including germination (Aprile et al. 2019), growth (Ci et al. 2010), respiration (Huybrechts et al. 2019), transpiration (Haider et al. 2021), water relations (Rady and Hemida 2015), nutrient uptake (Qin et al. 2020), photosynthesis (Nazar et al. 2012), and biomass accumulation (Ahmad et al. 2012). Several studies reported its detrimental effects on other plants at different growth stages (Peralta-Videa et al. 2002; Daud et al. 2015; Shamshad et al. 2018). The entry of Cd into plant cells induces oxidative stress, resulting from the increased production of reactive oxygen species (ROS), which leads to cell damage and reduced plant productivity (Lamhamdi et al. 2013). Plants have antioxidant systems that protect them from oxidation damage through detoxification mechanisms, which are highly compartmentalized and complex in plant cells (Chen et al. 2023).
Germination is the first process where a seed plant embryo begins to grow and develop into a seedling (Lei et al. 2021). It requires energy to occur and initiate a regulation of enzymatic reactions which activates cell metabolism (Seneviratne et al. 2019; Zhang et al. 2021). Seed germination is blocked if any of these functions are negatively impacted (Lamhamdi et al. 2013). One of the explanations recognized for HM’s impact on the physiology of plants is that they induce many nutrient disruptions (Qin et al. 2020). Storage material degradation is one of the most important processes that regulate the germination of seeds after imbibition with water (Liu et al. 2022). Several studies reported the toxic impact of HM on seed germination (Cokkizgin and Cokkizgin 2010; Stanković et al. 2010; Lei et al. 2021), however, few reported on Cd (Ahmad et al. 2012; Ling et al. 2015; Baruah et al. 2019). For instance, Ahmad et al. (2012) evaluated the impact of different Cd concentrations on seed germination and seedling growth of different wheat cultivars. According to the authors, Cd was toxic to seed germination and shoot and root growth, and these effects were enhanced at higher concentrations. In another study, Baruah et al. (2019) investigated the toxic effect of increasing levels of Cd on the germination and growth of wheat, tomato, and pea seedlings, and reported that tomato seeds were highly sensitive to Cd, followed by pea seeds and finally wheat seeds. The results of this study also indicated that wheat has a greater potential for contamination of the food chain if grown in polluted soil due to Cd’s high transfer and mobility.
Durum wheat is used to satisfy most dietary needs of large numbers of people. Its nutritional value is of great importance, as it is one of the few crops that is extensively grown as a staple food (Rehman Arif et al. 2020; Pehlivan Karakas et al. 2021; Zheng et al. 2023). In addition, this crop is cultivated in different zones of the world on diverse soils with variable toxic metal levels (Aprile et al. 2019). Cd is one of the HMs that accumulate in the grain of durum wheat to levels that exceed the standards set by the Codex Alimentarius Commission (FAO/WHO 2019). To the best of our knowledge, many studies investigated the effect of Cd on wheat (Ahmad et al. 2012; Abbas et al. 2017; Nawaz et al. 2021), however, fewer on durum wheat (Aprile et al. 2019). In light of the growing concern surrounding HM pollution and its potential impact on food security, this study focuses on Cd and its effects on durum wheat germination. Our hypothesis is that Cd significantly inhibits the germination and early growth of durum wheat, with varying impacts on different cultivars (Karim, Nachit, and Louiza). Therefore, the present paper aims at understanding the growth changes that occur during seed germination under HM stress. In this work, by studying the morphological effects of Cd during the germination phase of durum wheat seeds with assessment of the tolerance of three cultivars (Karim, Nachit, and Louiza) to Cd stress, we present the extent of Cd uptake by wheat seedlings and its influence on germination.
Materials and methods
Plant material and experimental site
The experiments were performed using seeds of three varieties of durum wheat (Triticum durum L. cv. Karim, cv. Nachit, cv. Louiza), provided by the National Institute for Agronomic Research (INRA), Rabat, Morocco. The experiments were conducted at the Faculty of Science and Techniques of Mohammedia, within the laboratory of Biochemistry, Environment & Agri-food (LBEA) in Mohammedia, Morocco.
Seed germination
The study aims to investigate the influence of increasing concentrations of Cd (0, 0.125, 0.25, 0.375, 0.5, 0.675, 0.75, 0.875, 1 g/L) on germination, biomass production, and shoot and root length. The Cd solutions were prepared by dissolving CdCl2, H2O in ultrapure water, and adjusting the pH of the solution to 5.5 with HNO3.
Wheat seeds with a homogeneous aspect were surface sterilized with 5% (v/v) sodium hypochlorite for 10 min then rinsed several times with distilled water. Forty seeds of each variety were placed in Petri dishes that contained two filter papers to maintain moisture. The dishes were treated with 6 mL of sterilized water for the control and the same volume of Cd aqueous solutions for the other treatments. Petri dishes were incubated in the darkness at 25 ± 2 °C and were arranged using a completely randomized design (CRD) in a factorial layout with three replications. After 7 days, the number of germinated seeds was determined.
Growth parameters’ measurement
After 7 days of germination, the shoot (SL) and root (RL) length were measured with a ruler based on the length of the shoots and the average of the main axial roots. Seedling samples were weighed to determine fresh weight (FW) while dry weight (DW) measurements were obtained after drying samples in an oven at 70 °C for 48 h. Vigor index (VI), growth inhibition (GI), and tolerance index (TI) parameters were calculated to assess the effect of Cd on the development and plant growth as follows:
-
VI was calculated using the following formula (Baruah et al. 2019):
-
\( VI = \left( {{\text{radicale}}\;{\text{length}} + {\text{plumule}}\;{\text{length}}} \right) \times {\text{germination}}\;{\text{percentage}} \)
-
GI was determined for shoot and root using the following formula (Ahmad et al. 2013):
-
\( {\text{GI}} = \frac{{{\text{Mean}}\;{\text{Shoot}}\;{\text{or}}\;{\text{Root}}\;{\text{length}}\;{\text{in}}\;{\text{control}} - {\text{Mean}}\;{\text{shoot}}\;{\text{or}}\;{\text{Root}}\;{\text{length}}\;{\text{in}}\;{\text{Cd}}\;{\text{solution}}}}{{{\text{Mean}}\;{\text{Shoot}}\;{\text{or}}\;{\text{Root}}\;{\text{length}}\;{\text{in}}\;{\text{control}}}} \)
-
TI was determined using the following formula (Iqbal and Rahmati 1992):
-
\( {\text{TI}} = \frac{{{\text{Mean}}\;{\text{root}}\;{\text{length}}\;{\text{in}}\;{\text{metal}}}}{{{\text{Mean}}\;{\text{root}}\;{\text{length}}\;{\text{in}}\;{\text{control}}}} \times 100 \)
Statistics analysis
Statistical analyses were conducted using IBM SPSS Statistics Data Editor. The one-way general linear model ANOVA was used to assess the impact of each treatment. The Tukey test was performed for post hoc testing to identify significant differences between treatment groups. A significance level of 0.05 was used for all tests. To further explore the data, a Pearson (n-1) correlation and principal component analysis (PCA) were conducted using XLSTAT-software v.2016. The correlation analysis examined relationships between variables, while PCA reduced data dimensionality and identified underlying patterns.
Results
Effect of Cd on seed germination percentage
Cd had a significant negative effect on wheat seed germination (Fig. 1). However, the inhibition of germination varied between cultivars. Seed germination inhibition was observed for Karim, Nachit, and Louiza at 0.625, 0.875, and 0.625 g/L of Cd, respectively. Karim and Louiza seed germination decreased from 0.125 to 0.625 g/L and were significantly (p < 0.05) reduced starting from 0.625 of g/L with a germination percentage around 69% which continued to decrease with the increase of Cd concentrations to reach 26.67 and 5% respectively under 1 g/L of Cd. In the case of Nachit, 0.875 g/L of Cd significantly (p < 0.001) reduced the germination percentage with a value of 24.17%. This reduction was maximum at 1 g/L of Cd with 21.67% of seeds germination.
Effect of Cd on root and shoot length and fresh and dry weights
The results showed that the negative effect of Cd was more evident in the early stages of seedling development compared to the germination stage where a significant (p < 0.05) inhibition was noticed in SL at 0.125 g/L of Cd for Karim and Louiza, respectively, and at 0.5 g/L of Cd for Nachit compared to the control (Table 1). Furthermore, a significant (p < 0.05) decline was recorded in RL at 0.25 g/L of Cd exposure for Karim, and 0.125 g/L of Cd for Nachit and Louiza compared to the control. The inhibition is enhanced at concentrations starting from 0.375 g/L of Cd for Karim, from 0.25 g/L of Cd for Nachit, and from 0.25 g/L of Cd for Louiza. Moreover, at 0.625, 0.875, and 0.375 g/L, Cd drastically inhibited RL for Karim, Nachit, and Louiza, respectively. Based on the highest Cd concentration, both SL and RL were negatively impacted in this order: Louiza < Nachit < Karim. On the other hand, Cd showed no significant effect on DW and FW for all the studied wheat cultivars compared to the control (Table 1).
Effect of Cd on wheat tolerance indices
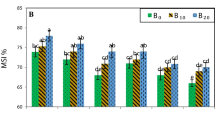
The effect of Cd on VI was found to be statistically highly significant (p < 0.001) according to Fig. 2. There was a notable variation among wheat cultivars in response to Cd, and this variation was also statistically significant (p < 0.001). At a concentration of 0.125 g/L of Cd, VI exhibited a drastic reduction. Specifically, for Karim, the decrease was 37.4%, for Nachit, it was 39.5%, and for Louiza, it was 54.94%. As the Cd concentrations increased to 0.375, 0.5, 0.625, 0.75, 0.875, and 1 g/L of Cd, Karim experienced a progressive decrease in VI with values of 74.82, 80.12, 89.30, 97.28, 99.03, and 99.1%, respectively. Similarly, Louiza exhibited a decline in VI with values of 87.32, 90.47, 91.75, 96.11, 99.26, and 99.97% for the corresponding Cd concentrations. In the case of Nachit, the VI showed a dose-dependent decrease where it notably declined at Cd concentrations of 0.875 and 1 g/L of Cd, reaching values of 99.47 and 99.56%, respectively (Fig. 2).
The response of wheat cultivars TI to increasing levels of Cd stress exhibited variability (Fig. 3). TI progressively decreased for all cultivars with increasing concentrations of Cd. Notably, significant reductions were observed for Nachit (41.37%) and Louiza (44.85%) at a low concentration of 0.125 g/L of Cd, and for Karim (37.70%) at 0.25 g/L of Cd. Despite a decrease in TI at the lowest Cd concentration, Nachit demonstrated superior tolerance to Cd stress under 0.375, 0.5, 0.625, and 0.75 g/L, with tolerance rates of 30.35, 26.16, 25.05, and 21.97%, respectively. In comparison to Nachit, Karim, and Louiza displayed lower TI of 19.36, 11.38, 6.17, 1.91%, and 8.15, 5.16, 4.00, 2.91%, respectively, during the same range of Cd concentrations. At high concentrations, Karim, Nachit, and Louiza exhibited minimal tolerance compared to the control (100%), with TI values of 1.27 and 1.09% for Karim, 0.66 and 0.65% for Nachit, and 1.43 and 0.12% for Louiza at 0.875 and 1 g/L of Cd, respectively. Overall, Nachit cultivar showed the highest TI values, followed by Karim and then Louiza.
The impact of Cd stress on root growth inhibition (RGI) was more pronounced compared to its effect on shoot growth inhibition (SGI) (Fig. 4). SGI was significantly increased for Karim and Louiza cultivars at the same concentration of Cd, while no significant change was recorded for Nachit cultivar in comparison to the control. On the other side, both Nachit and Louiza cultivars recorded significant increase of RGI at 0.125 g/L of Cd stress, while no significant effect was noticed for Karim cultivar. Increased significant (p < 0.001) values of SGI were observed at higher concentrations of 0.875 and 1 g/L of Cd compared to the control. Indeed, Karim and Louiza exhibited significant (p < 0.001) improved values of SGI starting from 0.125 g/L of Cd, with maximum values occurring at 0.875 and 1 g/L of Cd compared to the control. On the other hand, RGI was significantly affected in all cultivars starting from 0.125 g/L of Cd for Nachit and Louiza (p < 0.001), and at 0.25 g/L of Cd for Karim (p < 0.001). Among the cultivars, the order of increased SGI values was Nachit < Karim < Louiza, whereas for RGI, it was Karim < Nachit < Louiza.
Pearson correlation and principal component analysis
The correlation matrix reveals several noteworthy relationships among the variables (Table 2). One particularly important finding is the strong significant (p < 0.001) correlations observed between GP and six of the measured parameters. GP showed a significant positive correlation with SL (r = 0.826, p < 0.001), VI (r = 0.719, p < 0.001), TI (r = 0.655, p < 0.001), and RL (r = 0.644, p < 0.001) and a significant negative correlation with RGI (r = − 0.812, p < 0.001), and SGI (r = − 0.655, p < 0.001). Three significant positive correlations were recorded among the growth parameters. The strongest significant correlation (r = 0.862, p < 0.001) was observed between SL and RL, followed by the correlation between FW and DW (r = 0.620, p < 0.001), and then the correlation between SL and FW (r = 0.519, p < 0.05). These parameters, especially RL and SL showed strong correlations with the tolerance parameters where they recorded a significant positive correlation with VI (r = 0.986, p < 0.001 and r = 0.931, p < 0.001, respectively) and TI (r = 0.973, p < 0.001 and r = 0.858, p < 0.001, respectively) and a significant negative correlation with SGI (r = − 0.973, p < 0.001 and r = − 0.858, p < 0.001, respectively) and RGI (r = − 0.808, p < 0.001 and r = − 0.970, p < 0.001, respectively). Among tolerance traits, VI and TI showed a significant positive correlation (r = 0.968, p < 0.001) and recorded a significant negative correlation with SGI (r = − 0.968, p < 0.001 and r = − 1.000, p < 0.001, respectively) and RGI (r = − 0.886, p < 0.001 and r = − 0.801, p < 0.001, respectively). In addition, SGI and RGI showed a positive significant correlation (r = − 0.801, p < 0.001).
Principal component analysis (PCA) took into account all the analyzed parameters of the different treatments and showed that first and second components had a high cumulative variance (96.23% for Louiza, 90.27% for Karim, and 87.04% for Nachit) (Fig. 5). The biplot of the PCA analysis revealed that elevated Cd concentrations (0.75, 0.875, and 1 g/L of Cd) were positively associated with both SGI and RGI but especially with RGI for all wheat cultivars. In contrast, high Cd exposure led to a significant reduction in several parameters, including GP, SL, RL, FW, VI, and TI for all cultivars. Interestingly, the negative effect of Cd on Louiza growth and tolerance parameters (Fig. 5c) was observed even at medium concentrations (0.375, 0.5, 0.625 g/L) compared to Karim (Fig. 5a) and Nachit (Fig. 5b). Furthermore, the analysis demonstrated that Louiza DW was most reduced at higher Cd concentrations, whereas Karim and Nachit DW did not exhibit comparable susceptibility to the higher Cd concentrations in terms.
Principal component analysis of the studied traits of wheat under different Cd concentrations for three cultivars a Karim, b Nachit, and c Louiza. DW: Dry weight, FW: Fresh weight, GP: Germination percentage, RGI: Root growth inhibition, RL: Root length, SGI: Shoot growth inhibition, SL: Shoot length, TI: Tolerance index, VI: Vigor index
Discussion
Cd is a hazardous HM known to have severe effects on wheat and can cause various diseases in humans (Li et al. 2023). Therefore, further investigation into the toxicity of Cd in this essential crop is warranted to enhance our understanding of the underlying processes. Significant variations were observed among the studied cultivars (Karim, Nachit, and Louiza) treated with different concentrations (ranging from 0.125 to 1 g/L) of Cd. Our study revealed that the presence of Cd has suppressive effects on both seed germination and seedling growth. The same findings were reported by previous studies (Ali and Nas 2018; Safari et al. 2020) where Cd application reduced germination percentage and index, hampered root and shoot length, decreased tolerance levels, and negatively impacted the dry mass of both roots and shoots. In a recent study, Shedeed and Farahat (2023) analyzed the effects of two Cd concentrations (80 and 100 mg/L) on wheat seed germination and found that Cd decreased germination rates, increased radicle phytotoxicity, and reduced seedling biomass. In our study, Cd showed similar effects on wheat seeds. Furthermore, it significantly inhibited germination (p < 0.05) for Karim and Louiza at 0.625 g/L (69.17%) and for Nachit at 0.875 g/L (24.17%). It was explained that the inhibitory effect of Cd on seed germination and growth is mainly due to reduced water uptake, which hinders the growth of the seed embryo (Huybrechts et al. 2019; Haider et al. 2021). Moreover, the deactivation of starch degradation in the endosperm, results in impaired transfer of soluble sugars to the embryo axis of the seed, ultimately exacerbating nutrient deficiency in the embryo (Rahoui et al. 2015). There have been reports of a reduction in alpha-amylase activity as a result of a decrease in starch release from cotyledons under the influence of Cd stress (Kalai et al. 2016). FW and DW were not significantly affected, in our experiment, by the increasing concentrations of Cd, which was also reported in the study of Mahmood et al. (2009) where the biomass yield was not impacted by Cd stress. However, Cd significantly reduced shoot and root length for all cultivars. Several studies support our results showing that Cd adversely affected the normal growth of wheat and other plants by reducing seed germination and decreasing root and shoot length (Guilherme et al. 2015; Zhang et al. 2019; An et al. 2022; Lu et al. 2022; Zhou et al. 2023; Zhao et al. 2023). Root growth inhibition was more pronounced than shoot growth inhibition. It was explained that Cd stress has a more noticeable impact on durum wheat roots causing alterations in root structure, including changes in root morphology, disruption of the integrity of cell membranes and walls, reduced cell number, and altered cell shapes (Popa et al. 2020; Sabella et al. 2022). Additionally, it is known that the suppression of root elongation has been demonstrated as an initial and distinct manifestation of Cd toxicity (He et al. 2017). This inhibition may be also attributed to the fact that Cd stress can induce water deficit in seedlings (Seneviratne et al. 2019). Among the cultivars, the order of inhibition for shoot length was Nachit < Karim < Louiza, whereas for root length, it was Karim < Nachit < Louiza, which revealed that there parameters were differentially affected by Cd concentrations for the different cultivars. Furthermore, Cd application has been shown to inhibit seedling growth by disrupting the breakdown of storage proteins through interactions with proteolytic enzymes (Han et al. 2023). This, together with changes in protein patterns and reduced root respiration, leads to increased nitrite production and impaired cellular functions, further contributing to the retardation of seedling growth (Seneviratne et al. 2019; Popa et al. 2020; Zulfiqar et al. 2022a). This reduction in root size led to diminished viability of root cells and an increase in cell death, accompanied by the gradual production of ROS (Thind et al. 2021; Hasan et al. 2022). Furthermore, the decline in seedling growth can be attributed to the physiological limitations caused by Cd stress, which leads to a reduction in plant vigor and overall growth, depending on Cd dose (Rady and Hemida 2015; Carvalho et al. 2023). These findings are consistent with our results, as the vigor index showed a significant decrease (p < 0.001) under increased Cd concentrations; however, this effect showed discrepancies between different cultivars. Indeed, Nachit cultivar showed higher resistance toward Cd stress and higher germination rates for most treatments (0.125–0.75 g/L) compared to Karim and Louiza cultivars. This could potentially be attributed to intervarietal genetic variances, which confer either resistance or susceptibility to Cd stress (Ahmad et al. 2012). TI clearly revealed the response of the studied cultivars toward increasing Cd concentrations that not only reduced tolerance, but also inhibited plant growth. Nachit cultivar showed the highest TI values, followed by Karim and then Louiza. The diverse reactions of the different wheat cultivars to Cd stress indicate the existence of inherent resistance in some cultivars, while others may lack such resilience. Similarly to other parameters, the lower TIs observed in wheat cultivars under Cd stress can be linked to modifications in key physiological mechanisms. These include reduced uptake of water and nutrients, impaired enzymatic activities crucial for metabolism and energy production, hindered hormonal regulation, and elevated production of ROS (He et al. 2017; Qin et al. 2020; Haider et al. 2021; Zulfiqar et al. 2022a). These alterations occur during the growth stages of wheat plants and collectively contribute to the decreased tolerance toward Cd stress (Amirahmadi et al. 2020).
Conclusion
Our findings indicate that germination percentage, as well as root and shoot length of early stages of growth, serve as reliable indicators of Cd toxicity in wheat. Cadmium exhibited detrimental effects on wheat growth parameters even at low concentrations, with the highest level of inhibition observed at the highest applied concentration (1g/L). Nachit cv demonstrated superior performance, as evidenced by its leading TI values, while Louiza cv showed the lowest. Significant correlations are found among various growth and tolerance parameters. Notably, Nachit emerged as the most tolerant cultivar, suggesting its potential suitability for successful cultivation in Cd-contaminated soil. These findings emphasize the intricate relationship between Cd exposure and wheat development, calling for further investigations to unveil the underlying mechanisms of the differential tolerance of the studied cultivars to Cd toxicity also Cd contamination’s implications for food security and environmental health.
References
Abbas MS, Akmal M, Ullah S, Hassan MU, Farooq S (2017) Effectiveness of zinc and gypsum application against cadmium toxicity and accumulation in wheat (Triticum aestivum L.). Commun Soil Sci Plant Anal 48(14):1659–1668. https://doi.org/10.1080/00103624.2017.1373798
Ahmad I, Akhtar MJ, Zahir ZA, Jamil A (2012) Effect of cadmium on seed germination and seedling growth of four wheat (Triticum aestivum L.) cultivars. Pak J Bot 44(5):1569–1574
Ahmad I, Akhtar MJ, Asghar HN, Zahir ZA (2013) Comparative efficacy of growth media in causing cadmium toxicity to wheat at seed germination stage. Int J Agric Biol 15(3):1560–8530
Ahmad I, Gul I, Irum S, Manzoor M, Arshad M (2023) Accumulation of heavy metals in wild plants collected from the industrial sites—Potential for phytoremediation. Int J Environ Sci Technol 20(5):5441–5452. https://doi.org/10.1007/s13762-022-04340-3
Ali M, Nas FS (2018) The effect of lead on plants in terms of growing and biochemical parameters: a review. MOJ Ecol Environ Sci 3(4):265–268. https://doi.org/10.15406/mojes.2018.03.00098
Amirahmadi E, Mohammad Hojjati S, Kammann C, Ghorbani M, Biparva P (2020) The potential effectiveness of biochar application to reduce soil cd bioavailability and encourage oak seedling growth. Appl Sci 10(10):3410. https://doi.org/10.3390/app10103410
An T, Wu Y, Xu B, Zhang S, Deng X, Zhang Y, Siddique KH, Chen Y (2022) Nitrogen supply improved plant growth and Cd translocation in maize at the silking and physiological maturity under moderate Cd stress. Ecotoxicol Environ Saf 230:113137. https://doi.org/10.1016/j.ecoenv.2021.113137
Aprile A, Sabella E, Francia E, Milc J, Ronga D, Pecchioni N, Ferrari E, Luvisi A, Vergine M, De Bellis L (2019) Combined effect of cadmium and lead on durum wheat. Int J Mol Sci 20(23):5891. https://doi.org/10.3390/ijms20235891
Baruah N, Mondal SC, Farooq M, Gogoi N (2019) Influence of heavy metals on seed germination and seedling growth of wheat, pea, and tomato. Water Air Soil Pollut 230(12):273. https://doi.org/10.1007/s11270-019-4329-0
Carvalho MEA, Agathokleous E, Nogueira ML, Brunetto G, Brown PH, Azevedo RA (2023) Neutral-to-positive cadmium effects on germination and seedling vigor, with and without seed priming. J Hazard Mater 448:130813. https://doi.org/10.1016/j.jhazmat.2023.130813
Chen G, Wang J, He G, Li S, Li X, Tao X, Liang S, Deng F, Zeng F, Chen Z-H, Xu S (2023) Physiological and transcriptomic evidence of antioxidative system and ion transport in chromium detoxification in germinating seedlings of soybean. Environ Pollut 320:121047. https://doi.org/10.1016/j.envpol.2023.121047
Cheng M, Cui Y, Yan X, Zhang R, Wang J, Wang X (2022) Effect of dual-modified cassava starches on intelligent packaging films containing red cabbage extracts. Food Hydrocoll 124:107225. https://doi.org/10.1016/j.foodhyd.2021.107225
Ci D, Jiang D, Wollenweber B, Dai T, Jing Q, Cao W (2010) Cadmium stress in wheat seedlings: growth, cadmium accumulation and photosynthesis. Acta Physiol Plant 32(2):365–373. https://doi.org/10.1007/s11738-009-0414-0
Cokkizgin A, Cokkizgin H (2010) Effects of lead (PbCl2) stress on germination of lentil (Lens culinaris Medic.) lines. Afr J Biotechnol 9(50):8608–8612. https://doi.org/10.4314/ajb.v9i50
Daud MK, Quiling H, Lei M, Ali B, Zhu SJ (2015) Ultrastructural, metabolic and proteomic changes in leaves of upland cotton in response to cadmium stress. Chemosphere 120:309–320. https://doi.org/10.1016/j.chemosphere.2014.07.060
FAO/WHO (2019) Codex Alimentarius (2019) Codex general standard for contaminants and toxins in food and feed, CXS 193. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf.
Ge C, Ding Y, Wang Z, Wan D, Wang Y, Shang Q, Luo S (2009) Responses of wheat seedlings to cadmium, mercury and trichlorobenzene stresses. J Environ Sci 21(6):806–813. https://doi.org/10.1016/S1001-0742(08)62345-1
Gianazza E, Wait R, Sozzi A, Regondi S, Saco D, Labra M, Agradi E (2007) Growth and protein profile changes in Lepidium sativum L. plantlets exposed to cadmium. Environ Exp Bot 59(2):179–187. https://doi.org/10.1016/j.envexpbot.2005.12.005
Guilherme MDFS, Oliveira HM, Silva ED (2015) Cadmium toxicity on seed germination and seedling growth of wheat Triticum aestivum L. Acta Sci Biol Sci 37(4):499. https://doi.org/10.4025/actascibiolsci.v37i4.28148
Haider FU, Liqun C, Coulter JA, Cheema SA, Wu J, Zhang R, Wenjun M, Farooq M (2021) Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf 211:111887. https://doi.org/10.1016/j.ecoenv.2020.111887
Han Z, Osman R, Liu Y, Wei Z, Wang L, Xu M (2023) Analyzing the impacts of cadmium alone and in co-existence with polypropylene microplastics on wheat growth. Front Plant Sci 14:1240472. https://doi.org/10.3389/fpls.2023.1240472
Hasan N, Choudhary S, Laskar RA, Naaz N, Sharma N (2022) Comparative study of cadmium nitrate and lead nitrate [Cd(NO3)2 and Pb(NO3)2] stress in cyto-physiological parameters of Capsicum annuum L. Hortic Environ Biotechnol 63(5):627–641. https://doi.org/10.1007/s13580-021-00417-z
He S, Yang X, He Z, Baligar VC (2017) Morphological and physiological responses of plants to cadmium toxicity: a review. Pedosphere 27(3):421–438. https://doi.org/10.1016/S1002-0160(17)60339-4
Huybrechts M, Cuypers A, Deckers J, Iven V, Vandionant S, Jozefczak M, Hendrix S (2019) Cadmium and plant development: an agony from seed to seed. Int J Mol Sci 20(16):3971. https://doi.org/10.3390/ijms20163971
Iqbal MZ, Rahmati K (1992) Tolerance of Albizia lebbeck to Cu and Fe application. Ekológia ČSFR 11(4):427–430
Kalai T, Bouthour D, Manai J, Bettaieb Ben Kaab L, Gouia H (2016) Salicylic acid alleviates the toxicity of cadmium on seedling growth, amylases and phosphatases activity in germinating barley seeds. Arch Agron Soil Sci 62(6):892–904. https://doi.org/10.1080/03650340.2015.1100295
Khan A, Khan S, Khan MA, Qamar Z, Waqas M (2015) The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ Sci Pollut Res 22(18):13772–13799. https://doi.org/10.1007/s11356-015-4881-0
Lamhamdi M, El Galiou O, Bakrim A, Nóvoa-Muñoz JC, Arias-Estévez M, Aarab A, Lafont R (2013) Effect of lead stress on mineral content and growth of wheat (Triticum aestivum) and spinach (Spinacia oleracea) seedlings. Saudi J Biol Sci 20(1):29–36. https://doi.org/10.1016/j.sjbs.2012.09.001
Lei K, Sun S, Zhong K, Li S, Hu H, Sun C, Zheng Q, Tian Z, Dai T, Sun J (2021) Seed soaking with melatonin promotes seed germination under chromium stress via enhancing reserve mobilization and antioxidant metabolism in wheat. Ecotoxicol Environ Saf 220:112241. https://doi.org/10.1016/j.ecoenv.2021.112241
Li Y, Rahman SU, Qiu Z, Shahzad SM, Nawaz MF, Huang J, Naveed S, Li L, Wang X, Cheng H (2023) Toxic effects of cadmium on the physiological and biochemical attributes of plants, and phytoremediation strategies: a review. Environ Pollut 325:121433. https://doi.org/10.1016/j.envpol.2023.121433
Ling T, Meiying G, Jun R (2015) Effects of cadmium on seed germination, coleoptile growth, and root elongation of six pulses. Pol J Environ Stud 24(1):295–299. https://doi.org/10.15244/pjoes/29942
Liu X, Wang H, He F, Du X, Ren M, Bao Y (2022) The TaWRKY22–TaCOPT3D pathway governs cadmium uptake in wheat. Int J Mol Sci 23(18):10379. https://doi.org/10.3390/ijms231810379
Lu L, Zhai X, Li X, Wang S, Zhang L, Wang L, Jin X, Liang L, Deng Z, Li Z, Wang Y, Fu X, Hu H, Wang J, Mei Z, He Z, Wang F (2022) Met1-specific motifs conserved in OTUB subfamily of green plants enable rice OTUB1 to hydrolyse Met1 ubiquitin chains. Nat Commun 13(1):4672. https://doi.org/10.1038/s41467-022-32364-3
Mahmood T, Gupta KJ, Kaiser WM (2009) Cadmium stress stimulates nitric oxide production by wheat roots. Pak J Bot 41(3):1285–1290
Muramoto S, Aoyama I (1990) Effects of fertilizers on the vicissitude of cadmium in rice plant. J Environ Sci Health Part Environ Sci Eng Toxicol 25(6):629–635. https://doi.org/10.1080/10934529009375585
Nawaz H, Anwar-ul-Haq M, Akhtar J, Arfan M (2021) Cadmium, chromium, nickel and nitrate accumulation in wheat (Triticum aestivum L.) using wastewater irrigation and health risks assessment. Ecotoxicol Environ Saf 208:111685. https://doi.org/10.1016/j.ecoenv.2020.111685
Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NA (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci. https://doi.org/10.4236/ajps.2012.310178
Pehlivan Karakas F, Keskin CN, Agil F, Zencirci N (2021) Profiles of vitamin B and E in wheat grass and grain of einkorn (Triticum monococcum spp. monococcum), emmer (Triticum dicoccum ssp. dicoccum Schrank), durum (Triticum durum Desf.), and bread wheat (Triticum aestivum L.) cultivars by LC-ESI-MS/MS analysis. J Cereal Sci 98:103177. https://doi.org/10.1016/j.jcs.2021.103177
Peralta-Videa JR, Gardea-Torresdey JL, Gomez E, Tiemann KJ, Parsons JG, Carrillo G (2002) Effect of mixed cadmium, copper, nickel and zinc at different pHs upon alfalfa growth and heavy metal uptake. Environ Pollut 119(3):291–301. https://doi.org/10.1016/S0269-7491(02)00105-7
Popa C, Bratu AM, Bacalum M, Prepelita P (2020) Application of the laser technology on the effect of Cd phytotoxicity in the detection of NH3, C2H4, C2H5OH and CO2 biomolecules at Triticum aestivum plantlets. Sustain Chem Pharm 15:100208. https://doi.org/10.1016/j.scp.2019.100208
Qin S, Liu H, Nie Z, Rengel Z, Gao W, Li C, Zhao P (2020) Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: a review. Pedosphere 30(2):168–180. https://doi.org/10.1016/S1002-0160(20)60002-9
Rady MM, Hemida KA (2015) Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol Environ Saf 119:178–185. https://doi.org/10.1016/j.ecoenv.2015.05.008
Rahoui S, Chaoui A, Ben C, Rickauer M, Gentzbittel L, El Ferjani E (2015) Effect of cadmium pollution on mobilization of embryo reserves in seedlings of six contrasted Medicago truncatula lines. Phytochemistry 111:98–106. https://doi.org/10.1016/j.phytochem.2014.12.002
Rehman Arif MA, Attaria F, Shokat S, Akram S, Waheed MQ, Arif A, Börner A (2020) Mapping of QTLs associated with yield and yield related traits in durum wheat (Triticum durum desf) under irrigated and drought conditions. Int J Mol Sci 21(7):2372. https://doi.org/10.3390/ijms21072372
Sabella E, Aprile A, Tenuzzo BA, Carata E, Panzarini E, Luvisi A, De Bellis L, Vergine M (2022) Effects of cadmium on root morpho-physiology of durum wheat. Front Plant Sci 13:936020. https://doi.org/10.3389/fpls.2022.936020
Sabiha-Javied SN, Waheed S, Uz Zaman Q, Aslam A, Tufail M, Nasir R (2023) Uptake of heavy metal in wheat from application of different phosphorus fertilizers. J Food Compos Anal 115:104958. https://doi.org/10.1016/j.jfca.2022.104958
Safari M, Kari Dolatabad H, Ndu U, Rivera NA (2020) Protective effect of Pseudomonas spp. Isolates and zinc on seed germination and β-amylase activity in wheat cultivars under cadmium stress. Acta Physiol Plant 42(4):50. https://doi.org/10.1007/s11738-020-03038-8
Seneviratne M, Rajakaruna N, Rizwan M, Madawala HMSP, Ok YS, Vithanage M (2019) Heavy metal-induced oxidative stress on seed germination and seedling development: a critical review. Environ Geochem Health 41(4):1813–1831. https://doi.org/10.1007/s10653-017-0005-8
Shahid M, Dumat C, Khalid S, Niazi NK, Antunes PMC (2017) Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. In: De Voogt P (ed) Reviews of environmental contamination and toxicology, vol 241. Springer International Publishing, Cham, pp 73–137
Shamshad S, Shahid M, Rafiq M, Khalid S, Dumat C, Sabir M, Murtaza B, Farooq ABU, Shah NS (2018) Effect of organic amendments on cadmium stress to pea: a multivariate comparison of germinating vs young seedlings and younger vs older leaves. Ecotoxicol Environ Saf 151:91–97. https://doi.org/10.1016/j.ecoenv.2018.01.002
Shedeed ZA, Farahat EA (2023) Alleviating the toxic effects of Cd and Co on the seed germination and seedling biochemistry of wheat (Triticum aestivum L.) using Azolla pinnata. Environ Sci Pollut Res 30(30):76192–76203. https://doi.org/10.1007/s11356-023-27566-1
Stanković M, Topuzović M, Marković A, Pavlović D, Đelić G, Bojović B, Branković S (2010) Influence of zinc (ZN) on germination of wheat (Triticum Aestivum L.). Biotechnol Biotechnol Equip 24(sup1):236–239. https://doi.org/10.1080/13102818.2010.10817842
Thind S, Hussain I, Ali S, Rasheed R, Ashraf MA (2021) Silicon application modulates growth, physio-chemicals, and antioxidants in wheat (Triticum aestivum L.) exposed to different cadmium regimes. Dose-Response 19(2):155932582110146. https://doi.org/10.1177/15593258211014646
Zhang F, Liu M, Li Y, Che Y, Xiao Y (2019) Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci Total Environ 655:1150–1158. https://doi.org/10.1016/j.scitotenv.2018.11.317
Zhang Q, Cheng Z, Wang Y, Fu L (2021) Dietary protein-phenolic interactions: characterization, biochemical-physiological consequences, and potential food applications. Crit Rev Food Sci Nutr 61(21):3589–3615. https://doi.org/10.1080/10408398.2020.1803199
Zhao S, Kamran M, Rizwan M, Ali S, Yan L, Alwahibi MS, Elshikh MS, Riaz M (2023) Regulation of proline metabolism, AsA-GSH cycle, cadmium uptake and subcellular distribution in Brassica napus L. under the effect of nano-silicon. Environ Pollut 335:122321. https://doi.org/10.1016/j.envpol.2023.122321
Zheng H, Fan X, Bo W, Yang X, Tjahjadi T, Jin S (2023) A multiscale point-supervised network for counting maize tassels in the wild. Plant Phenomics 5:0100. https://doi.org/10.34133/plantphenomics.0100
Zhou P, Zhang P, He M, Cao Y, Adeel M, Shakoor N, Jiang Y, Zhao W, Li Y, Li M, Azeem I, Jia L, Rui Y, Ma X, Lynch I (2023) Iron-based nanomaterials reduce cadmium toxicity in rice (Oryza sativa L.) by modulating phytohormones, phytochelatin, cadmium transport genes and iron plaque formation. Environ Pollut 320:121063. https://doi.org/10.1016/j.envpol.2023.121063
Zulfiqar U, Ayub A, Hussain S, Waraich EA, El-Esawi MA, Ishfaq M, Ahmad M, Ali N, Maqsood MF (2022a) Cadmium toxicity in plants: recent progress on morpho-physiological effects and remediation strategies. J Soil Sci Plant Nutr 22(1):212–269. https://doi.org/10.1007/s42729-021-00645-3
Zulfiqar U, Jiang W, Xiukang W, Hussain S, Ahmad M, Maqsood MF, Ali N, Ishfaq M, Kaleem M, Haider FU, Farooq N, Naveed M, Kucerik J, Brtnicky M, Mustafa A (2022b) Cadmium phytotoxicity, tolerance, and advanced remediation approaches in agricultural soils. A Compr Rev Front Plant Sci 13:773815. https://doi.org/10.3389/fpls.2022.773815
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology were performed by NZJ, GD, AH, and MAEM; formal analysis was conducted by NZJ, OA, and FM; data analysis was done by NZJ, MAEM, OA, and IA; writing and original draft preparation were provided by NZJ; review and editing were revised by NZJ, MAEM, AH, and IA; supervision was analyzed by AH and MAEM.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Communicated by Mian Abdur Rehman Arif.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaari Jabri, N., Ait-El-Mokhtar, M., Mekkaoui, F. et al. Impacts of cadmium toxicity on seed germination and seedling growth of Triticum durum cultivars. CEREAL RESEARCH COMMUNICATIONS (2023). https://doi.org/10.1007/s42976-023-00467-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42976-023-00467-2