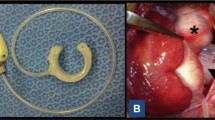
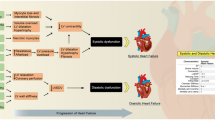
Abstract
Our understanding of the complex pathophysiology of Heart failure with preserved ejection fraction (HFpEF) is limited by the lack of a robust in vivo model. Existing in-vivo models attempt to reproduce the four main phenotypes of HFpEF; ageing, obesity, diabetes mellitus and hypertension. To date, there is no in vivo model that represents all the haemodynamic characteristics of HFpEF, and only a few have proven to be reliable for the preclinical evaluation of potentially new therapeutic targets. HFpEF accounts for 50% of all the heart failure cases and its incidence is on the rise, posing a huge economic burden on the health system. Patients with HFpEF have limited therapeutic options available. The inadequate effectiveness of current pharmaceutical therapeutics for HFpEF has prompted the development of device-based treatments that target the hemodynamic changes to reduce the symptoms of HFpEF. However, despite the potential of device-based solutions to treat HFpEF, most of these therapies are still in the developmental stage and a relevant HFpEF in vivo model will surely expedite their development process. This review article outlines the major limitations of the current large in-vivo models in use while discussing how these designs have helped in the development of therapy devices for the treatment of HFpEF.
Graphical abstract



Similar content being viewed by others
Abbreviations
- cGMP:
-
Cyclic guanosine monophosphate
- DOCA:
-
Deoxy-corticosterone acetate
- EF:
-
Ejection fraction
- HF:
-
Heart failure
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- IASD:
-
Interatrial shunt device
- LAAD:
-
Left atrial assist device
- LA:
-
Left atrium
- LAP:
-
Left atrial pressure
- LV:
-
Left ventricular
- LVADs:
-
Left ventricular assist devices
- LVDD:
-
Left ventricular diastolic dysfunction
- LVEDP:
-
Left ventricular end-diastolic pressure
- LVH:
-
Left ventricular hypertrophy
- LVPO:
-
Left ventricular pressure-overload
- MCS:
-
Mechanical circulatory support
- NHP:
-
Non-human primate
- RVHT:
-
Renovascular hypertension
- SGLT2:
-
Sodium-glucose cotransporter-2
- TTE:
-
Transthoracic echocardiography
References
National Heart LaBI (2021) Cardiovascular disease is on the rise, but we know how to curb it. We’ve done it before. Available from https://www.nhlbi.nih.gov/news/2021/cardiovascular-disease-rise-we-know-how-curb-it-weve-done-it
Barry A Borlaug WSC (2022) Treatment and prognosis of heart failure with preserved ejection fraction. UpToDate
Gevaert AB, Boen JRA, Segers VF, Van Craenenbroeck EM (2019) Heart failure with preserved ejection fraction: a review of cardiac and noncardiac pathophysiology. Front Physiol 10:638
Gazewood JD, Turner PL (2017) Heart failure with preserved ejection fraction: diagnosis and management. Am Fam Physician 96(9):582–588
Oktay AA, Rich JD, Shah SJ (2013) The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep 10(4):401–410
Tromp J, Shen L, Jhund PS, Anand IS, Carson PE, Desai AS et al (2019) Age-related characteristics and outcomes of patients with heart failure with preserved ejection fraction. J Am Coll Cardiol 74(5):601–612
Guazzi M, Ghio S, Adir Y (2020) Pulmonary hypertension in HFpEF and HFrEF: JACC review topic of the week. J Am Coll Cardiol 76(9):1102–1111
Clinic M (2015) Heart failure with preserved ejection fraction (HFpEF): more than diastolic dysfunction. Available from https://www.mayoclinic.org/medical-professionals/cardiovascular-diseases/news/heart-failure-with-preserved-ejection-fraction-hfpef-more-than-diastolic-dysfunction/mac-20430055
Guazzi M (2014) Pulmonary hypertension in heart failure preserved ejection fraction: prevalence, pathophysiology, and clinical perspectives. Circ Heart Fail 7(2):367–377
Zamfirescu MB, Ghilencea LN, Popescu MR, Bejan GC, Maher SM, Popescu AC, Dorobanțu M (2021) The E/e’Ratio—role in risk stratification of acute heart failure with preserved ejection fraction. Medicina 57(4):375
Conceição G, Heinonen I, Lourenço AP, Duncker DJ, Falcão-Pires I (2016) Animal models of heart failure with preserved ejection fraction. Neth Heart J 24(4):275–286
Charles CJ, Rademaker MT, Scott NJ, Richards AM (2020) Large animal models of heart failure: reduced vs. preserved ejection fraction. Animals 10(10):1906
Miyagi C, Miyamoto T, Kuroda T, Karimov JH, Starling RC, Fukamachi K (2022) Large animal models of heart failure with preserved ejection fraction. Heart Fail Rev 27(2):595–608
Sharp TE 3rd, Scarborough AL, Li Z, Polhemus DJ, Hidalgo HA, Schumacher JD et al (2021) Novel Göttingen miniswine model of heart failure with preserved ejection fraction integrating multiple comorbidities. JACC Basic Transl Sci 6(2):154–170
Charles CJ, Lee P, Li RR, Yeung T, Ibraham Mazlan SM, Tay ZW et al (2020) A porcine model of heart failure with preserved ejection fraction: magnetic resonance imaging and metabolic energetics. ESC Heart Fail 7(1):92–102
Li H, Xia YY, Xia CL, Li Z, Shi Y, Li XB et al (2022) Mimicking metabolic disturbance in establishing animal models of heart failure with preserved ejection fraction. Front Physiol 13:879214
van Ham WB, Kessler EL, Oerlemans MI, Handoko ML, Sluijter JP, van Veen TA, den Ruijter HM, de Jager SC (2022) Clinical phenotypes of heart failure with preserved ejection fraction to select preclinical animal models. Basic Transl Sci 7(8):844–857
Olver TD, Edwards JC, Jurrissen TJ, Veteto AB, Jones JL, Gao C et al (2019) Western diet-fed, aortic-banded Ossabaw swine: a preclinical model of cardio-metabolic heart failure. JACC Basic Transl Sci 4(3):404–421
Silva KAS, Emter CA (2020) Large animal models of heart failure: a translational bridge to clinical success. JACC Basic Transl Sci 5(8):840–856
Samson R, Jaiswal A, Ennezat PV, Cassidy M, Le Jemtel TH (2016) Clinical phenotypes in heart failure with preserved ejection fraction. J Am Heart Assoc 5(1):e002477
Sasayama S, Ross J Jr, Franklin D, Bloor CM, Bishop S, Dilley RB (1976) Adaptations of the left ventricle to chronic pressure overload. Circ Res 38(3):172–178
Koide M, Nagatsu M, Zile MR, Hamawaki M, Swindle MM, Keech G et al (1997) Premorbid determinants of left ventricular dysfunction in a novel model of gradually induced pressure overload in the adult canine. Circulation 95(6):1601–1610
Fujii AM, Aoyagi T, Flanagan MF, Takahashi T, Bennett-Guerrero E, Colan SD et al (1993) Response of the hypertrophied left ventricle to tachycardia: importance of maturation. Am J Physiol 264(3 Pt 2):H983–H993
Ye Y, Gong G, Ochiai K, Liu J, Zhang J (2001) High-energy phosphate metabolism and creatine kinase in failing hearts: a new porcine model. Circulation 103(11):1570–1576
Gelpi RJ, Park M, Gao S, Dhar S, Vatner DE, Vatner SF (2011) Apoptosis in severe, compensated pressure overload predominates in nonmyocytes and is related to the hypertrophy but not function. Am J Physiol Heart Circ Physiol 300(3):H1062–H1068
Song LS, Pi Y, Kim SJ, Yatani A, Guatimosim S, Kudej RK et al (2005) Paradoxical cellular Ca2+ signaling in severe but compensated canine left ventricular hypertrophy. Circ Res 97(5):457–464
Neeb ZP, Edwards JM, Alloosh M, Long X, Mokelke EA, Sturek M (2010) Metabolic syndrome and coronary artery disease in Ossabaw compared with Yucatan swine. Comp Med 60(4):300–315
Bikou O, Watanabe S, Hajjar RJ, Ishikawa K (2018) A pig model of myocardial infarction: catheter-based approaches. Methods Mol Biol 1816:281–294
Yarbrough WM, Mukherjee R, Stroud RE, Rivers WT, Oelsen JM, Dixon JA et al (2012) Progressive induction of left ventricular pressure overload in a large animal model elicits myocardial remodeling and a unique matrix signature. J Thorac Cardiovasc Surg 143(1):215–223
Emter CA, Tharp DL, Ivey JR, Ganjam VK, Bowles DK (2011) Low-intensity interval exercise training attenuates coronary vascular dysfunction and preserves Ca2+-sensitive K+ current in miniature swine with LV hypertrophy. Am J Physiol Heart Circ Physiol 301(4):H1687–H1694
Emter CA, Baines CP (2010) Low-intensity aerobic interval training attenuates pathological left ventricular remodeling and mitochondrial dysfunction in aortic-banded miniature swine. Am J Physiol Heart Circ Physiol 299(5):H1348–H1356
Wallner M, Eaton DM, Berretta RM, Borghetti G, Wu J, Baker ST et al (2017) A feline HFpEF model with pulmonary hypertension and compromised pulmonary function. Sci Rep 7(1):16587
Zhen N, Loo SJ, Su LP, Tao ZH, Gui F, Luo JH et al (2021) A diastolic dysfunction model in non-human primates with transverse aortic constriction. Exp Anim 70(4):498–507
Gyöngyösi M, Pavo N, Lukovic D, Zlabinger K, Spannbauer A, Traxler D et al (2017) Porcine model of progressive cardiac hypertrophy and fibrosis with secondary postcapillary pulmonary hypertension. J Transl Med 15(1):202
Munagala VK, Hart CY, Burnett JC Jr, Meyer DM, Redfield MM (2005) Ventricular structure and function in aged dogs with renal hypertension: a model of experimental diastolic heart failure. Circulation 111(9):1128–1135
Hamdani N, Bishu KG, von Frieling-Salewsky M, Redfield MM, Linke WA (2013) Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res 97(3):464–471
Borlaug BA, Carter RE, Melenovsky V, DeSimone CV, Gaba P, Killu A et al (2017) Percutaneous pericardial resection: a novel potential treatment for heart failure with preserved ejection fraction. Circ Heart Fail 10(4):e003612
Nachar W, Merlet N, Maafi F, Shi Y, Mihalache-Avram T, Mecteau M et al (2019) Cardiac inflammation and diastolic dysfunction in hypercholesterolemic rabbits. PLoS ONE 14(8):e0220707
van den Dorpel MMP, Heinonen I, Snelder SM, Vos HJ, Sorop O, van Domburg RT et al (2018) Early detection of left ventricular diastolic dysfunction using conventional and speckle tracking echocardiography in a large animal model of metabolic dysfunction. Int J Cardiovasc Imaging 34(5):743–749
Qian C, Gong L, Yang Z, Chen W, Chen Y, Xu Z et al (2015) Diastolic dysfunction in spontaneous type 2 diabetes rhesus monkeys: a study using echocardiography and magnetic resonance imaging. BMC Cardiovasc Disord 15:59
Shapiro BP, Owan TE, Mohammed S, Kruger M, Linke WA, Burnett JC Jr et al (2008) Mineralocorticoid signaling in transition to heart failure with normal ejection fraction. Hypertension 51(2):289–295
Sorop O, Heinonen I, van Kranenburg M, van de Wouw J, de Beer VJ, Nguyen ITN et al (2018) Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc Res 114(7):954–964
Schwarzl M, Hamdani N, Seiler S, Alogna A, Manninger M, Reilly S et al (2015) A porcine model of hypertensive cardiomyopathy: implications for heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 309(9):H1407–H1418
Shah SJ, Borlaug BA, Kitzman DW, McCulloch AD, Blaxall BC, Agarwal R et al (2020) Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute working group summary. Circulation 141(12):1001–1026
van de Wouw J, Steenhorst JJ, Sorop O, van Drie RWA, Wielopolski PA, Kleinjan A et al (2021) Impaired pulmonary vasomotor control in exercising swine with multiple comorbidities. Basic Res Cardiol 116(1):51
Miyagi C, Miyamoto T, Karimov JH, Starling RC, Fukamachi K (2021) Device-based treatment options for heart failure with preserved ejection fraction. Heart Fail Rev 26(4):749–762
Rosalia L, Ozturk C, Shoar S, Fan Y, Malone G, Cheema FH et al (2021) Device-based solutions to improve cardiac physiology and hemodynamics in heart failure with preserved ejection fraction. JACC Basic Transl Sci 6(9–10):772–795
Burlacu A, Simion P, Nistor I, Covic A, Tinica G (2019) Novel percutaneous interventional therapies in heart failure with preserved ejection fraction: an integrative review. Heart Fail Rev 24(5):793–803
Wintrich J, Kindermann I, Ukena C, Selejan S, Werner C, Maack C et al (2020) Therapeutic approaches in heart failure with preserved ejection fraction: past, present, and future. Clin Res Cardiol 109(9):1079–1098
Del Buono MG, Iannaccone G, Scacciavillani R, Carbone S, Camilli M, Niccoli G et al (2020) Heart failure with preserved ejection fraction diagnosis and treatment: an updated review of the evidence. Prog Cardiovasc Dis 63(5):570–584
Sun W, Zou H, Yong Y, Liu B, Zhang H, Lu J et al (2022) The RAISE trial: a novel device and first-in-man trial. Circ Heart Fail 15(4):e008362
Nishikawa T, Saku K, Uike K, Uemura K, Sunagawa G, Tohyama T et al (2020) Prediction of haemodynamics after interatrial shunt for heart failure using the generalized circulatory equilibrium. ESC Heart Fail 7(5):3075–3085
Feld Y, Reisner Y, Meyer-Brodnitz G, Hoefler R (2023) The CORolla device for energy transfer from systole to diastole: a novel treatment for heart failure with preserved ejection fraction. Heart Fail Rev 28(2):307–314
Silverman DN, Shah SJ (2019) Treatment of heart failure with preserved ejection fraction (HFpEF): the phenotype-guided approach. Curr Treat Options Cardiovasc Med 21(4):20
Ning B, Zhang F, Song X, Hao Q, Li Y, Li R et al (2020) Cardiac contractility modulation attenuates structural and electrical remodeling in a chronic heart failure rabbit model. J Int Med Res 48(10):030006052096291
Zucker IH, Hackley JF, Cornish KG, Hiser BA, Anderson NR, Kieval R et al (2007) Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension 50(5):904–910
Schüttler D, Bapat A, Kääb S, Lee K, Tomsits P, Clauss S et al (2020) Animal models of atrial fibrillation. Circ Res 127(1):91–110
Snyder T, Cornat F, Jem N, Pruvost R, Benoit S, Monticone E et al (2022) CARD19: In vitro and in vivo testing of the pulsatile CorWave membrane LVAD. ASAIO J 68(Supplement 2):54
Miyagi C, Kuban BD, Flick CR, Polakowski AR, Miyamoto T, Karimov JH, Starling RC, Fukamachi K (2023) Left atrial assist device for heart failure with preserved ejection fraction: initial results with torque control mode in diastolic heart failure model. Heart Fail Rev 28(2):287–296
Roh J, Hill JA, Singh A, Valero-Muñoz M, Sam F (2022) Heart failure with preserved ejection fraction: heterogeneous syndrome, diverse preclinical models. Circ Res 130(12):1906–1925
Lourenço AP, Leite-Moreira AF, Balligand J-L, Bauersachs J, Dawson D, De Boer RA et al (2018) An integrative translational approach to study heart failure with preserved ejection fraction: a position paper from the Working Group on Myocardial Function of the European Society of Cardiology. Eur J Heart Fail 20(2):216–227
Malone A, Gallagher S, Saidi J, Rizq G, O’Dowd E, Vallence D, Hameed A (2022) In vitro benchtop mock circulatory loop for heart failure with preserved ejection fraction emulation. Front Cardiovasc Med 9:910120
Funding
Dr Aamir Hameed would like to acknowledge Enterprise Ireland for their support through their Commercialisation Fund (CF-2019-1136-P). This funding supports the development of a novel device-based solution for HFpEF. This work has been carried out at the RCSI University of Medicine and Health Sciences.
Author information
Authors and Affiliations
Contributions
AH conceived the study design. SMF, AM, ZR and TA wrote the first manuscript draft. FHC, AM, BH, NA, JS, MA and JO’N critically reviewed the manuscript drafts. All authors reviewed and approved the final manuscript. AH and JO’N are the co-senior and co-corresponding authors.
Corresponding authors
Ethics declarations
Competing interests
Aamir Hameed (CMO), Andrew Malone (CSO) and Faisal H. Cheema (Non-Executive Director) are associated with a Startup, an RCSI and Tissue Engineering Research Group (TERG) spinout, Pumpinheart Ltd., which is developing a novel device for HFpEF. Jim O'Neill is on the advisory board of Pumpinheart Ltd. The rest of the authors do not have any known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Heart failure with preserved ejection fraction (HFpEF) continues to plague the elderly population and those with pre-existing comorbidities, exorbitantly contributing towards the increasing cardiovascular disease burden within this population.
• However, animal models for studying HFpEF remain scarce due, in large part, to the complex interplay of pathophysiology and co-existing comorbidities, rendering it mechanistically difficult to curate optimal models.
• Additionally, translating HFpEF phenotypes into animal models remains a complicated process since both the developing triggers and the diagnostic approaches vary between humans and animals.
• The present review paper comprehensively evaluates novel models, such as the left ventricular apex balloon insertion and progressive LVPO models, which have both significantly contributed to the development of a more robust and reliable HFpEF model.
• Further research on large animal models is warranted to truly elucidate their utility in therapy device development.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fisher, S.M., Murally, A.R., Rajabally, Z. et al. Large animal models to study effectiveness of therapy devices in the treatment of heart failure with preserved ejection fraction (HFpEF). Heart Fail Rev 29, 257–276 (2024). https://doi.org/10.1007/s10741-023-10371-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-023-10371-w