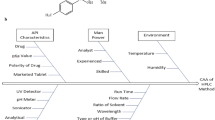
Abstract
Bovine clinical mastitis has significant repercussions for farmers across the globe. Meloxicam, a COX-2 inhibitor, attenuates mastitis symptoms and is also approved for veterinary use. An RP-HPLC (Reverse Phase-High Performance Liquid Chromatography) method development and validation is essential in the pharmaceutical industry to assess API (Active Pharmaceutical Ingredient) quantity present in the pharmaceutical dosage forms. RP-HPLC method of meloxicam was developed and optimized with the aid of QbD (Quality by Design) using Box-Behnken design (BBD). The pH of the aqueous mobile phase, acetonitrile (ACN) percentage, and column temperature were chosen as influence variables, and retention time (RT) and tailing factor (Tf) were selected as response variables. The optimum experimental conditions were selected as pH ~ 3 of the aqueous mobile phase, 65% v/v ACN, and 30˚C as column temperature. The drug was eluted at 6.02 min RT with 1.18 as Tf. The method was subjected to validation for accuracy, linearity, precision, range, sensitivity, and robustness and was found to comply with ICH Q2 (R1) guidelines. The in vitro bioequivalent studies were performed in hydrochloric acid, pH ~ 1.2; acetate buffer, pH ~ 4.5; and phosphate buffer, pH ~ 6.8 for two veterinary brands of meloxicam tablets, and their release profiles were compared by mathematical models. Both the brands, brand 1 and 2 exhibited significant (Unpaired t-test, P < 0.05) differences in dissolution efficiency (DE) and mean dissolution time (MDT) except DE at pH 1.2. However, brands 1 and 2 showed similarity (f2 > 50) in terms of release of meloxicam except at pH 6.8 (f2 = 47.01). The in vitro release of meloxicam followed Peppas kinetics except for brand 2 at pH 6.8, where it followed the Higuchi model. Moreover, the recovery of meloxicam extracted with ACN in the milk sample was estimated to be 99.67 ± 0.58% significantly (Unpaired t-test, P < 0.05) higher than 90.34 ± 6.77% extracted with methanol. In conclusion, the optimized and validated RP-HPLC method of meloxicam may further be used for the analysis of drug content in pharmaceutical dosage forms in addition to biological fluids.
Graphical abstract





Similar content being viewed by others
Data availability
Data will be made available on request.
References
A. de la Concha-Bermejillo, J. Romano, Pregnancy loss in cattle. Clin. Theriogenology 13(3), 167–180 (2021). https://doi.org/10.58292/CT.V13.9334
S. McDougall, M.A. Bryan, R.M. Tiddy, Effect of treatment with the nonsteroidal anti-inflammatory meloxicam on milk production, somatic cell count, probability of re-treatment, and culling of dairy cows with mild clinical mastitis. J. Dairy Sci. 92(9), 4421–4431 (2009). https://doi.org/10.3168/JDS.2009-2284
van S. FJS, A. E, M. S, and H. H, (2018) “Addition of meloxicam to the treatment of bovine clinical mastitis results in a net economic benefit to the dairy farmer. J Dairy Sci 101 (4) 3387–3397 https://doi.org/10.3168/JDS.2017-12869.
M.O. Caldeira, R.M. Bruckmaier, O. Wellnitz, Effects of local or systemic administration of meloxicam on mammary gland inflammatory responses to lipopolysaccharide-induced mastitis in dairy cows. J. Dairy Sci. 104(1), 1039–1052 (2021). https://doi.org/10.3168/JDS.2020-18691
V.G.S.S. Jyothi et al., Film forming topical dermal spray of meloxicam attenuated pain and inflammation in carrageenan-induced paw oedema in Sprague Dawley rats. J. Drug Deliv. Sci. Technol. 70, 103195 (2022). https://doi.org/10.1016/J.JDDST.2022.103195
P. Jedziniak, T. Szprengier-Juszkiewicz, M. Olejnik, In-house reference materials: 5-hydroxyflunixin and meloxicam in cow milk—preparation and evaluation. Anal. Chim. Acta 637(1–2), 346–350 (2009). https://doi.org/10.1016/J.ACA.2008.10.060
J.C. Mucklow, Martindale: the complete drug reference. Br. J. Clin. Pharmacol. 49(6), 613–613 (2000). https://doi.org/10.1046/j.1365-2125.2000.00206.x
N.Y. Khalil, K.F. Aldosari, Meloxicam. Profiles Drug Subst. Excipients Relat. Methodol. 45, 159–197 (2020). https://doi.org/10.1016/BS.PODRM.2019.10.006
L. Subbiah et al., Development of Meloxicam-chitosan magnetic nanoconjugates for targeting rheumatoid arthritis joints: Pharmaceutical characterization and preclinical assessment on murine models. J. Magn. Magn. Mater. 523, 167571 (2021). https://doi.org/10.1016/J.JMMM.2020.167571
F.Y. Han, D.A. Brockman, J.R. Nicholson, L. Corradini, M.T. Smith, “Pharmacological characterization of the chronic phase of the monoiodoacetate-induced rat model of osteoarthritis pain in the knee joint. Clin. Exp. Pharmacol. Physiol (2021). https://doi.org/10.1111/1440-1681.13551
A.D. Kaye et al., “The role of exparel plus meloxicam for postoperative pain management. Curr. Pain Headache Reports 24(3), 1–7 (2020). https://doi.org/10.1007/S11916-020-0837-2
M. Starek and J. Krzek, (2009) “A review of analytical techniques for determination of oxicams, nimesulide and nabumetone,” Talanta, vol. 77, no. 3. Elsevier, 925–942 15 https://doi.org/10.1016/j.talanta.2008.09.022.
N. Salunkhe, N. Jadhav, S. Bhinge, Meloxicam quantification in rabbit plasma by RP-HPLC: optimization and application to pharmacokinetic study. Futur J Pharm Sci (2020). https://doi.org/10.1186/S43094-020-00069-3
C. Zhan, C. Wang, Y. Wang, H. Xie, and J. C.-B., “Using stable isotope labeled internal standard for LC‐MS/MS quantitation of meloxicam in human plasma,” Wiley Online Libr., Accessed: Jul. 28, 2021. [Online]. Available: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/bmc.5217
D. Salazar-Rojas, A. Intilangelo, S.E. Vignaduzzo, R.M. Maggio, Development and validation of a green method for dissolution monitoring of pharmaceutical combinations. Meloxican and pridinol. J. Pharm. Biomed. Anal. 170, 228–233 (2019). https://doi.org/10.1016/J.JPBA.2019.03.038
A.H. Nadim, M.A. Al-Ghobashy, M. Nebsen, M.A. Shehata, Optimization of photocatalytic degradation of meloxicam using titanium dioxide nanoparticles: application to pharmaceutical wastewater analysis, treatment, and cleaning validation. Environ. Sci. Pollut. Res. (2015). https://doi.org/10.1007/S11356-015-4713-2
C. Zhan et al., Using stable isotope labeled internal standard for LC-MS/MS quantitation of meloxicam in human plasma. Biomed. Chromatogr. (2021). https://doi.org/10.1002/BMC.5217
N.K. Sahoo, M. Sahu, P.S. Rao, N. Sandhya Rani, J.N.V. Indira Devi, G. Ghosh, Validation of assay indicating method development of Meloxicam in bulk and some of its tablet dosage forms by RP-HPLC. Springerplus 3(1), 2014 (2014). https://doi.org/10.1186/2193-1801-3-95
ICH Q8(R2), International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use pharmaceutical development q8 (r2). 2009.
R. N. Dash, M. Habibuddin, T. Humaira, and A. A. Patel, (2015) “Application of Quality by Design for the Optimization of an HPLC Method to Determine Ezetimibe in a Supersaturable Self-Nanoemulsifying Formulation 38 (8) 874–885 https://doi.org/10.1080/10826076.2014.982867.
A. Jain, S. Beg, S. Saini, T. Sharma, O.P. Katare, B. Singh, Application of chemometric approach for QbD-enabled development and validation of an RP-HPLC method for estimation of methotrexate. J. Liq. Chromatogr. Relat. Technol. 42(15–16), 502–512 (2019). https://doi.org/10.1080/10826076.2019.1626742
G.E.P. Box, D.W. Behnken, Some new three level designs for the study of quantitative variables. Technometrics 2(4), 455–475 (1960). https://doi.org/10.1080/00401706.1960.10489912
P.K. Sahu, C.S. Patro, “Application of chemometric response surface methodology in development and optimization of a RP-HPLC method for the separation of metaxalone and its base hydrolytic impuritiES. J Liquid Chromatograp Relat Tech 37(17), 2444–2464 (2014)
J.W. Bae, M.J. Kim, C.G. Jang, S.Y. Lee, Determination of meloxicam in human plasma using a HPLC method with UV detection and its application to a pharmacokinetic study. J. Chromatogr. B 859(1), 69–73 (2007). https://doi.org/10.1016/J.JCHROMB.2007.09.004
ICH Q2 (R1), “ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology Step 5 note for guidance on validation of analytical procedures: text and methodology (cpmp/ich/381/95) approval by cpmp november 1994 date for coming into operation,” 1995. Accessed: Jul. 05, 2021. [Online]. Available: http://www.emea.eu.int
S. B. Jadhav, P. S. Reddy, K. L. Narayanan, and P. N. Bhosale, (2017) Development of RP-HPLC, stability indicating method for degradation products of linagliptin in presence of metformin HCL by applying 2 level factorial design; and identification of impurity-VII VIII and IX and synthesis of impurity-VII. Sci Pharm 85 (3) https://doi.org/10.3390/SCIPHARM85030025.
26 USP, (2003) “The United Stated Pharmacopeia. Rockville, MD,” United States Pharmacopeial Conv. Inc, pp. 2155–2156.
M. Cerea et al., Non-uniform drug distribution matrix system (NUDDMat) for zero-order release of drugs with different solubility. Int. J. Pharm. 581, 119217 (2020). https://doi.org/10.1016/J.IJPHARM.2020.119217
A. Jain, S.K. Jain, In vitro release kinetics model fitting of liposomes: an insight. Chem. Phys. Lipids 201, 28–40 (2016). https://doi.org/10.1016/J.CHEMPHYSLIP.2016.10.005
I.Y. Wu, S. Bala, N. Škalko-Basnet, M.P. di Cagno, Interpreting non-linear drug diffusion data: utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 138, 105026 (2019). https://doi.org/10.1016/J.EJPS.2019.105026
A.M. Moydeen, M.S. Ali Padusha, E.F. Aboelfetoh, S.S. Al-Deyab, M.H. El-Newehy, Fabrication of electrospun poly(vinyl alcohol)/dextran nanofibers via emulsion process as drug delivery system: Kinetics and in vitro release study. Int. J. Biol. Macromol (2018). https://doi.org/10.1016/J.IJBIOMAC.2018.05.130
X. Xu, M. Al-Ghabeish, Y.S.R. Krishnaiah, Z. Rahman, M.A. Khan, Kinetics of drug release from ointments: role of transient-boundary layer. Int. J. Pharm. 494(1), 31–39 (2015). https://doi.org/10.1016/J.IJPHARM.2015.07.077
C.G. Varelas, D.G. Dixon, C.A. Steiner, Zero-order release from biphasic polymer hydrogels. J. Control. Release 34(3), 185–192 (1995). https://doi.org/10.1016/0168-3659(94)00085-9
N.V. Mulye, S.J. Turco, A simple model based on first order kinetics to explain release of highly water soluble drugs from porous dicalcium phosphate dihydrate matrices. Drug Dev. Ind. Pharm. 21(8), 943–953 (1995). https://doi.org/10.3109/03639049509026658
W.I. Higuchi, Analysis of data on the medicament release from ointments. J. Pharm. Sci. 51(8), 802–804 (1962). https://doi.org/10.1002/jps.2600510825
S.J. Desai, P. Singh, A.P. Simonelli, W.I. Higuchi, Investigation of factors influencing release of solid drug dispersed in inert matrices III. Quantitative studies involving the polyethylene plastic matrix. J. Pharm. Sci. 55(11), 1230–1234 (1966). https://doi.org/10.1002/jps.2600551113
N.A. Peppas, Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 60(4), 110–111 (1985)
A.W. Hixson, J.H. Crowell, Dependence of reaction velocity upon surface and Agitation: I—theoretical consideration. Ind. Eng. Chem. 23(8), 923–931 (1931). https://doi.org/10.1021/ie50260a018
P.J. Niebergall, G. Milosovich, J.E. Goyan, Dissolution rate studies II. Dissolution of particles under conditions of rapid agitation. J. Pharm. Sci. 52(3), 236–241 (1963). https://doi.org/10.1002/jps.2600520310
D.A. Diaz, S.T. Colgan, C.S. Langer, N.T. Bandi, M.D. Likar, L. Van Alstine, “Dissolution Similarity Requirements: How Similar or Dissimilar Are the Global Regulatory Expectations? AAPS J. 18(1), 15–22 (2015). https://doi.org/10.1208/S12248-015-9830-9
H.H. Moore, J.W. Flanner, Mathematical comparison of dissolution profiles. Pharm Technol 20, 64–74 (1996)
T. Yi Tsong, P. Sathe. Hammerstrom, V.P. Shah, Statistical assessment of mean differences between two dissolution data sets”. Ther Innov Regul Sci. 30(4), 1105–1112 (1996). https://doi.org/10.1177/009286159603000427
K.A. Khan, The concept of dissolution efficiency. J. Pharm. Pharmacol. 27(1), 48–49 (1975). https://doi.org/10.1111/j.2042-7158.1975.tb09378.x
K.A. Chatzizaharia, D.T. Hatziavramidis, Dissolution efficiency and design space for an oral pharmaceutical product in tablet form. Ind. Eng. Chem. Res. 54(24), 6305–6310 (2015). https://doi.org/10.1021/IE5050567
E. Dubreil-Chéneau, Y. Pirotais, M. Bessiral, B. Roudaut, E. Verdon, Development and validation of a confirmatory method for the determination of 12 non steroidal anti-inflammatory drugs in milk using liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1218(37), 6292–6301 (2011). https://doi.org/10.1016/J.CHROMA.2011.06.006
P. Gallo et al., Confirmatory analysis of nonsteroidal anti-inflammatory drugs in bovine milk by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A 1217(17), 2832–2839 (2010). https://doi.org/10.1016/J.CHROMA.2010.02.047
E.M. Malone, G. Dowling, C.T. Elliott, D.G. Kennedy, L. Regan, Development of a rapid, multi-class method for the confirmatory analysis of anti-inflammatory drugs in bovine milk using liquid chromatography tandem mass spectrometry. J. Chromatogr. A 1216(46), 8132–8140 (2009). https://doi.org/10.1016/J.CHROMA.2009.04.078
Ewp, “Committee for medicinal products for human use (CHMP) guideline on the investigation of bioequivalence discussion in the joint efficacy and quality working group adoption rev. 1 by chmp for release for consultation end of consultation rev. 1 (deadline for comments),” 1997, Accessed: Jul. 28, 2021. [Online]. Available: http://www.ema.europa.eu
P. Luger, K. Daneck, W. Engel, G. Trummlitz, K. Wagner, Structure and physicochemical properties of meloxicam, a new NSAID. Eur. J. Pharm. Sci. 4(3), 175–187 (1996). https://doi.org/10.1016/0928-0987(95)00046-1
WHO, “Technical Report Series No. 937, Annex 7, Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish Interchangeability.,” 2006.
Acknowledgements
This work was supported with funds from the National Institute of Pharmaceutical Education and Research, Hyderabad, Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, New Delhi, India
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jyothi, V.G.S.S., Veerabomma, H., Katta, C. et al. Computational quality-by-design strategy to validate high-performance liquid chromatography method for the estimation of meloxicam in bulk dosage forms and milk samples. ANAL. SCI. 40, 249–261 (2024). https://doi.org/10.1007/s44211-023-00448-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-023-00448-9