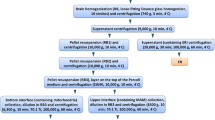
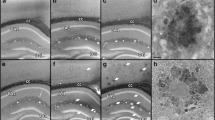
Abstract
Most scholars believe that amyloid-beta (Aβ) has the potential to induce apoptosis, stimulate an inflammatory cascade, promote oxidative stress and exacerbate the pathological progression of Alzheimer's disease (AD). Therefore, it is crucial to investigate the deposition of Aβ in AD. At approximately 6 months of age, APP/PS1 double transgenic mice gradually exhibit the development of plaques, as well as spatial and learning impairment. Notably, the hippocampus is specifically affected in the course of AD. Herein, 6-month-old APP/PS1 double transgenic mice were utilized, and the differentially expressed (DE) proteins in the hippocampus were identified and analyzed using 4D label-free quantitative proteomics technology and parallel reaction monitoring (PRM). Compared to wild-type mice, 29 proteins were upregulated and 25 proteins were downregulated in the AD group. Gene Ontology (GO) enrichment analysis of biological processes (BP) indicated that the DE proteins were mainly involved in ‘ribosomal large subunit biogenesis’. Molecular function (MF) analysis results were primarily associated with ‘5.8S rRNA binding’ and ‘structural constituent of ribosome’. In terms of cellular components (CC), the DE proteins were mainly found in ‘polysomal ribosome’, ‘cytosolic large ribosomal subunit’, ‘cytosolic ribosome’, and ‘large ribosomal subunit’, among others. Furthermore, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis demonstrated that the results were mainly enriched in the ‘Ribosome signaling pathway’. The key target proteins identified were ribosomal protein (Rp)l18, Rpl17, Rpl19, Rpl24, Rpl35, and Rpl6. The PRM verification results were consistent with the findings of the 4D label-free quantitative proteomics analysis. Overall, these findings suggest that Rpl18, Rpl17, Rpl19, Rpl24, Rpl35, and Rpl6 may have potential therapeutic value for the treatment of AD by targeting Aβ.






Similar content being viewed by others
Data availability
All data used to support the findings of this study are included within the article.
Code availability
Not applicable.
Abbreviations
- AA-tRNA:
-
Aminoacyl-tRNA
- Aβ:
-
Amyloid-beta
- AD:
-
Alzheimer's disease
- AGC:
-
Automatic gain control
- APOE:
-
Apolipoprotein E
- APP:
-
Amyloid precursor protein
- BACE-1:
-
Beta-secretase 1
- BP:
-
Biological processes
- CC:
-
Cellular Component
- ChEIs:
-
Cholinesterase inhibitors
- DE:
-
Differentially expressed
- ELISA:
-
Enzyme linked immunosorbent assay
- FDA:
-
Food and Drug Administration
- FDR:
-
False-positive rate
- GFAP:
-
Glial fibrillary acid protein
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- KOG:
-
EuKaryotic Orthologous Groups
- LC‒MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- MF:
-
Molecular function
- MMSE:
-
Minimum Mental State Examination
- NFTs:
-
Neurofibrillary tangles
- PCA:
-
Principal components analysis
- PPI:
-
Protein‒protein interaction
- PRM:
-
Parallel reaction monitoring
- Rp:
-
Ribosomal protein
- RSD:
-
Relative standard deviation
- SP:
-
Senile plaques
- TCM:
-
Traditional Chinese medicine
References
Anisimova AS, Meerson MB, Gerashchenko MV, Kulakovskiy IV, Dmitriev SE, Gladyshev VN (2020) Multifaceted deregulation of gene expression and protein synthesis with age. Proc Natl Acad Sci U S A 117:15581–15590. https://doi.org/10.1073/pnas.2001788117
Association A (2016) 2016 alzheimer’s disease facts and figures. Alzheimers Dement 12:459–509. https://doi.org/10.1016/j.jalz.2016.03.001
Awad D, Prattes M, Kofler L, Rossler I, Loibl M, Pertl M, Zisser G, Wolinski H, Pertschy B, Bergler H (2019) Inhibiting Eukaryotic Ribosome Biogenesis. Bmc Biol 17:46. https://doi.org/10.1186/s12915-019-0664-2
Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E (2011) Alzheimer’s disease. Lancet 377:1019–1031. https://doi.org/10.1016/S0140-6736(10)61349-9
Beason-Held LL, Goh JO, An Y, Kraut MA, O’Brien RJ, Ferrucci L, Resnick SM (2013) Changes in brain function occur years before the onset of cognitive impairment. J Neurosci 33:18008–18014. https://doi.org/10.1523/JNEUROSCI.1402-13.2013
Bertram L, Tanzi RE (2019) Alzheimer disease risk genes: 29 and counting. Nat Rev Neurol 15:191–192. https://doi.org/10.1038/s41582-019-0158-4
Biever A, Valjent E, Puighermanal E (2015) Ribosomal protein s6 phosphorylation in the nervous system: from regulation to function. Front Mol Neurosci 8:75. https://doi.org/10.3389/fnmol.2015.00075
Brandman O, Hegde RS (2016) Ribosome-associated protein quality control. Nat Struct Mol Biol 23:7–15. https://doi.org/10.1038/nsmb.3147
Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of alzheimer’s disease. Alzheimers Dement 3:186–191. https://doi.org/10.1016/j.jalz.2007.04.381
Brookmeyer R, Evans DA, Hebert L, Langa KM, Heeringa SG, Plassman BL, Kukull WA (2011) National estimates of the prevalence of alzheimer’s disease in the united states. Alzheimers Dement 7:61–73. https://doi.org/10.1016/j.jalz.2010.11.007
Bu XL, Sun BL, Wang YJ (2021) Prevention and treatment of Alzheimer′s disease: challenges and perspectives. Chin J Neurol 54:635–639. https://doi.org/10.3760/cma.j.cn113694-20210222-00129
Budd HS, Aisen PS, Barkhof F, Chalkias S, Chen T, Cohen S, Dent G, Hansson O, Harrison K, von Hehn C, Iwatsubo T, Mallinckrodt C, Mummery CJ, Muralidharan KK, Nestorov I, Nisenbaum L, Rajagovindan R, Skordos L, Tian Y, van Dyck CH, Vellas B, Wu S, Zhu Y, Sandrock A (2022) Two randomized phase 3 studies of aducanumab in early alzheimer’s disease. J Prev Alzheimers Dis 9:197–210. https://doi.org/10.14283/jpad.2022.30
Buhr F, Jha S, Thommen M, Mittelstaet J, Kutz F, Schwalbe H, Rodnina MV, Komar AA (2016) Synonymous codons direct cotranslational folding toward different protein conformations. Mol Cell 61:341–351. https://doi.org/10.1016/j.molcel.2016.01.008
Bursac S, Brdovcak MC, Donati G, Volarevic S (2014) Activation of the tumor suppressor p53 upon impairment of ribosome biogenesis. Biochim Biophys Acta 1842:817–830. https://doi.org/10.1016/j.bbadis.2013.08.014
Chen GQ, Ham Y (2018) Preclinical Alzheimer′s disease: emergence, challenge and thinking. Chin J Neurol 51:75–78. https://doi.org/10.3760/cma.j.issn.1006-7876.2018.01.017
Chinese Society of Dementia and Cognitive Impairment (2022) Chinese expert consensus on the diagnosis and treatment of mild cognitive impairment due to Alzheimer′s disease 2021. Chin J Neurol 55:421–440. https://doi.org/10.3760/cma.j.cn113694-20211004-00679
Choe YJ, Park SH, Hassemer T, Korner R, Vincenz-Donnelly L, Hayer-Hartl M, Hartl FU (2016) Failure of rqc machinery causes protein aggregation and proteotoxic stress. Nature 531:191–195. https://doi.org/10.1038/nature16973
Ciryam P, Tartaglia GG, Morimoto RI, Dobson CM, Vendruscolo M (2013) Widespread aggregation and neurodegenerative diseases are associated with supersaturated proteins. Cell Rep 5:781–790. https://doi.org/10.1016/j.celrep.2013.09.043
Chinese Dementia and Cognitive Disorders Writing Group, and Cognitive Disorders Professional Committee of Neurology Branch of Chinese Medical Doctor Association (2018) 2018 Chinese Guidelines for the Diagnosis and Treatment of Dementia and Cognitive Disorders (2) : Guidelines for the Diagnosis and treatment of Alzheimer's Disease. Natl Med J China 98:971-977. https://doi.org/10.3760/cma.j.issn.0376-2491.2018.13.004
Cummings JL, Cohen S, van Dyck CH, Brody M, Curtis C, Cho W, Ward M, Friesenhahn M, Rabe C, Brunstein F, Quartino A, Honigberg LA, Fuji RN, Clayton D, Mortensen D, Ho C, Paul R (2018) Abby: a phase 2 randomized trial of crenezumab in mild to moderate alzheimer disease. Neurology 90:e1889–e1897. https://doi.org/10.1212/WNL.0000000000005550
De Strooper B, Karran E (2016) The cellular phase of alzheimer’s disease. Cell 164:603–615. https://doi.org/10.1016/j.cell.2015.12.056
Dhikav V, Duraiswamy S, Anand KS (2017) Correlation between hippocampal volumes and medial temporal lobe atrophy in patients with alzheimer’s disease. Ann Indian Acad Neurol 20:29–35. https://doi.org/10.4103/0972-2327.199903
Egan MF, Kost J, Tariot PN, Aisen PS, Cummings JL, Vellas B, Sur C, Mukai Y, Voss T, Furtek C, Mahoney E, Harper ML, Vandenberghe R, Mo Y, Michelson D (2018) Randomized trial of verubecestat for mild-to-moderate alzheimer’s disease. N Engl J Med 378:1691–1703. https://doi.org/10.1056/NEJMoa1706441
Egan MF, Mukai Y, Voss T, Kost J, Stone J, Furtek C, Mahoney E, Cummings JL, Tariot PN, Aisen PS, Vellas B, Lines C, Michelson D (2019) Further analyses of the safety of verubecestat in the phase 3 epoch trial of mild-to-moderate alzheimer’s disease. Alzheimers Res Ther 11:68. https://doi.org/10.1186/s13195-019-0520-1
Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M (2005) Global prevalence of dementia: a delphi consensus study. Lancet 366:2112–2117. https://doi.org/10.1016/S0140-6736(05)67889-0
Fillit H, Green A (2021) Aducanumab and the fda - where are we now? Nat Rev Neurol 17:129–130. https://doi.org/10.1038/s41582-020-00454-9
Galton CJ, Gomez-Anson B, Antoun N, Scheltens P, Patterson K, Graves M, Sahakian BJ, Hodges JR (2001) Temporal lobe rating scale: application to alzheimer’s disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry 70:165–173. https://doi.org/10.1136/jnnp.70.2.165
Griciuc A, Tanzi RE (2021) The role of innate immune genes in alzheimer’s disease. Curr Opin Neurol 34:228–236. https://doi.org/10.1097/WCO.0000000000000911
Hamed M, Gladbach Y, Moller S, Fischer S, Ernst M, Struckmann S, Storch A, Fuellen G (2018) A workflow for the integrative transcriptomic description of molecular pathology and the suggestion of normalizing compounds, exemplified by parkinson’s disease. Sci Rep 8:7937. https://doi.org/10.1038/s41598-018-25754-5
Hardy J, De Strooper B (2017) Alzheimer’s disease: where next for anti-amyloid therapies? Brain 140:853–855. https://doi.org/10.1093/brain/awx059
Heggland I, Kvello P, Witter MP (2019) Electrophysiological characterization of networks and single cells in the hippocampal region of a transgenic rat model of alzheimer's disease. Eneuro 6. https://doi.org/10.1523/ENEURO.0448-17.2019.
Hey JA, Kocis P, Hort J, Abushakra S, Power A, Vyhnalek M, Yu JY, Tolar M (2018a) Correction to: discovery and identification of an endogenous metabolite of tramiprosate and its prodrug alz-801 that inhibits beta amyloid oligomer formation in the human brain. CNS Drugs 32:1185. https://doi.org/10.1007/s40263-018-0585-6
Hey JA, Yu JY, Versavel M, Abushakra S, Kocis P, Power A, Kaplan PL, Amedio J, Tolar M (2018b) Clinical pharmacokinetics and safety of alz-801, a novel prodrug of tramiprosate in development for the treatment of alzheimer’s disease. Clin Pharmacokinet 57:315–333. https://doi.org/10.1007/s40262-017-0608-3
Hull M, Sadowsky C, Arai H, Le Prince LG, Holstein A, Booth K, Peng Y, Yoshiyama T, Suzuki H, Ketter N, Liu E, Ryan JM (2017) Long-term extensions of randomized vaccination trials of acc-001 and qs-21 in mild to moderate alzheimer’s disease. Curr Alzheimer Res 14:696–708. https://doi.org/10.2174/1567205014666170117101537
Imbimbo BP, Ippati S, Watling M, Imbimbo C (2023) Role of monomeric amyloid-beta in cognitive performance in alzheimer’s disease: insights from clinical trials with secretase inhibitors and monoclonal antibodies. Pharmacol Res 187:106631. https://doi.org/10.1016/j.phrs.2022.106631
Jack CJ, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R (2018) Nia-aa research framework: toward a biological definition of alzheimer’s disease. Alzheimers Dement 14:535–562. https://doi.org/10.1016/j.jalz.2018.02.018
Jahn TR, Radford SE (2008) Folding versus aggregation: polypeptide conformations on competing pathways. Arch Biochem Biophys 469:100–117. https://doi.org/10.1016/j.abb.2007.05.015
Jia L, Du Y, Chu L, Zhang Z, Li F, Lyu D, Li Y, Li Y, Zhu M, Jiao H, Song Y, Shi Y, Zhang H, Gong M, Wei C, Tang Y, Fang B, Guo D, Wang F, Zhou A, Chu C, Zuo X, Yu Y, Yuan Q, Wang W, Li F, Shi S, Yang H, Zhou C, Liao Z, Lv Y, Li Y, Kan M, Zhao H, Wang S, Yang S, Li H, Liu Z, Wang Q, Qin W, Jia J (2020) Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in china: a cross-sectional study. Lancet Public Health 5:e661–e671. https://doi.org/10.1016/S2468-2667(20)30185-7
Juszkiewicz S, Chandrasekaran V, Lin Z, Kraatz S, Ramakrishnan V, Hegde RS (2018) Znf598 is a quality control sensor of collided ribosomes. Mol Cell 72:469–481. https://doi.org/10.1016/j.molcel.2018.08.037
Kudla G, Murray AW, Tollervey D, Plotkin JB (2009) Coding-sequence determinants of gene expression in escherichia coli. Science 324:255–258. https://doi.org/10.1126/science.1170160
Lacosta AM, Pascual-Lucas M, Pesini P, Casabona D, Perez-Grijalba V, Marcos-Campos I, Sarasa L, Canudas J, Badi H, Monleon I, San-Jose I, Munuera J, Rodriguez-Gomez O, Abdelnour C, Lafuente A, Buendia M, Boada M, Tarraga L, Ruiz A, Sarasa M (2018) Safety, tolerability and immunogenicity of an active anti-abeta(40) vaccine (abvac40) in patients with alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase i trial. Alzheimers Res Ther 10:12. https://doi.org/10.1186/s13195-018-0340-8
Li Y, Yu H, Chen C, Li S, Zhang Z, Xu H, Zhu F, Liu J, Spencer PS, Dai Z, Yang X (2020) Proteomic profile of mouse brain aging contributions to mitochondrial dysfunction, dna oxidative damage, loss of neurotrophic factor, and synaptic and ribosomal proteins. Oxid Med Cell Longev 2020:5408452. https://doi.org/10.1155/2020/5408452
Li SC, Han C, Qin YL, Zhao YF, Wei WY, Yang J, Shuai YY, Guo D (2022) A review of experimental animal models of Alzheimer’s disease. Acta Lab Anim Sci Sin 30:131–145. https://doi.org/10.3969/j.issn.1005-4847.2022.01.017
Loera-Valencia R, Cedazo-Minguez A, Kenigsberg PA, Page G, Duarte AI, Giusti P, Zusso M, Robert P, Frisoni GB, Cattaneo A, Zille M, Boltze J, Cartier N, Buee L, Johansson G, Winblad B (2019) Current and emerging avenues for alzheimer’s disease drug targets. J Intern Med 286:398–437. https://doi.org/10.1111/joim.12959
Lu H, Zhu YF, Xiong J, Wang R, Jia Z (2015) Potential extra-ribosomal functions of ribosomal proteins in saccharomyces cerevisiae. Microbiol Res 177:28–33. https://doi.org/10.1016/j.micres.2015.05.004
Lyu J, Wang Y, Mao J, Yao Y, Wang S, Zheng Y, Ye M (2018) Pseudotargeted ms method for the sensitive analysis of protein phosphorylation in protein complexes. Anal Chem 90:6214–6221. https://doi.org/10.1021/acs.analchem.8b00749
Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429:761–766. https://doi.org/10.1038/nature02617
McGeer PL, McGeer EG (2013) The amyloid cascade-inflammatory hypothesis of alzheimer disease: implications for therapy. Acta Neuropathol 126:479–497. https://doi.org/10.1007/s00401-013-1177-7
Meier F, Brunner AD, Koch S, Koch H, Lubeck M, Krause M, Goedecke N, Decker J, Kosinski T, Park MA, Bache N, Hoerning O, Cox J, Rather O, Mann M (2018) Online parallel accumulation-serial fragmentation (pasef) with a novel trapped ion mobility mass spectrometer. Mol Cell Proteomics 17:2534–2545. https://doi.org/10.1074/mcp.TIR118.000900
Mintun MA, Wessels AM, Sims JR (2021) Donanemab in early alzheimer’s disease. Reply N Engl J Med 385:667. https://doi.org/10.1056/NEJMc2109455
Neumann U, Ufer M, Jacobson LH, Rouzade-Dominguez ML, Huledal G, Kolly C, Luond RM, Machauer R, Veenstra SJ, Hurth K, Rueeger H, Tintelnot-Blomley M, Staufenbiel M, Shimshek DR, Perrot L, Frieauff W, Dubost V, Schiller H, Vogg B, Beltz K, Avrameas A, Kretz S, Pezous N, Rondeau JM, Beckmann N, Hartmann A, Vormfelde S, David OJ, Galli B, Ramos R, Graf A, Lopez LC (2018) The bace-1 inhibitor cnp520 for prevention trials in alzheimer's disease. Embo Mol Med 10. https://doi.org/10.15252/emmm.201809316.
Novak G, Streffer JR, Timmers M, Henley D, Brashear HR, Bogert J, Russu A, Janssens L, Tesseur I, Tritsmans L, Van Nueten L, Engelborghs S (2020) Long-term safety and tolerability of atabecestat (jnj-54861911), an oral bace1 inhibitor, in early alzheimer’s disease spectrum patients: a randomized, double-blind, placebo-controlled study and a two-period extension study. Alzheimers Res Ther 12:58. https://doi.org/10.1186/s13195-020-00614-5
Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T, Ashford E, Retout S, Hofmann C, Delmar P, Klein G, Andjelkovic M, Dubois B, Boada M, Blennow K, Santarelli L, Fontoura P (2017) A phase iii randomized trial of gantenerumab in prodromal alzheimer’s disease. Alzheimers Res Ther 9:95. https://doi.org/10.1186/s13195-017-0318-y
Ostrowitzki S, Bittner T, Sink KM, Mackey H, Rabe C, Honig LS, Cassetta E, Woodward M, Boada M, van Dyck CH, Grimmer T, Selkoe DJ, Schneider A, Blondeau K, Hu N, Quartino A, Clayton D, Dolton M, Dang Y, Ostaszewski B, Sanabria-Bohorquez SM, Rabbia M, Toth B, Eichenlaub U, Smith J, Honigberg LA, Doody RS (2022) Evaluating the safety and efficacy of crenezumab vs placebo in adults with early alzheimer disease: two phase 3 randomized placebo-controlled trials. Jama Neurol 79:1113–1121. https://doi.org/10.1001/jamaneurol.2022.2909
Paroni G, Bisceglia P, Seripa D (2019) Understanding the amyloid hypothesis in alzheimer’s disease. J Alzheimers Dis 68:493–510. https://doi.org/10.3233/JAD-180802
Pechmann S, Frydman J (2013) Evolutionary conservation of codon optimality reveals hidden signatures of cotranslational folding. Nat Struct Mol Biol 20:237–243. https://doi.org/10.1038/nsmb.2466
Pontecorvo MJ, Lu M, Burnham SC, Schade AE, Dage JL, Shcherbinin S, Collins EC, Sims JR, Mintun MA (2022) Association of donanemab treatment with exploratory plasma biomarkers in early symptomatic alzheimer disease: a secondary analysis of the trailblazer-alz randomized clinical trial. Jama Neurol 79:1250–1259. https://doi.org/10.1001/jamaneurol.2022.3392
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013) The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 9:63–75. https://doi.org/10.1016/j.jalz.2012.11.007
Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT (2016) Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther 8:23. https://doi.org/10.1186/s13195-016-0188-8
Pugazhenthi S (2017) Metabolic syndrome and the cellular phase of alzheimer’s disease. Prog Mol Biol Transl Sci 146:243–258. https://doi.org/10.1016/bs.pmbts.2016.12.016
Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jaggi F, Wolburg H, Gengler S, Haass C, Ghetti B, Czech C, Holscher C, Mathews PM, Jucker M (2006) Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. Embo Rep 7:940–946. https://doi.org/10.1038/sj.embor.7400784
Radio NM, Mundy WR (2008) Developmental neurotoxicity testing in vitro: models for assessing chemical effects on neurite outgrowth. Neurotoxicology 29:361–376. https://doi.org/10.1016/j.neuro.2008.02.011
Rafii MS, Sol O, Mobley WC, Delpretti S, Skotko BG, Burke AD, Sabbagh MN, Yuan SH, Rissman RA, Pulsifer M, Evans C, Evans AC, Beth G, Fournier N, Gray JA, Dos SA, Hliva V, Vukicevic M, Kosco-Vilbois M, Streffer J, Pfeifer A, Feldman HH (2022) Safety, tolerability, and immunogenicity of the aci-24 vaccine in adults with down syndrome: a phase 1b randomized clinical trial. Jama Neurol 79:565–574. https://doi.org/10.1001/jamaneurol.2022.0983
Reardon S (2023) Fda approves alzheimer’s drug lecanemab amid safety concerns. Nature 613:227–228. https://doi.org/10.1038/d41586-023-00030-3
Ricciarelli R, Fedele E (2017) The amyloid cascade hypothesis in alzheimer’s disease: it’s time to change our mind. Curr Neuropharmacol 15:926–935. https://doi.org/10.2174/1570159X15666170116143743
Sarazin M, Dorothee G, de Souza LC, Aucouturier P (2013) Immunotherapy in alzheimer’s disease: do we have all the pieces of the puzzle? Biol Psychiatry 74:329–332. https://doi.org/10.1016/j.biopsych.2013.04.011
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chetelat G, Teunissen CE, Cummings J, van der Flier WM (2021) Alzheimer’s disease. Lancet 397:1577–1590. https://doi.org/10.1016/S0140-6736(20)32205-4
Sheng SL (2004) Research Progress on Alzheimer′s Disease: Pathogenesis and Medical Therapy. Acta Acad Med Sin 02:101–103. https://doi.org/10.3969/j.issn.1009-0959.2003.06.005
Simms CL, Yan LL, Zaher HS (2017) Ribosome collision is critical for quality control during no-go decay. Mol Cell 68:361–373. https://doi.org/10.1016/j.molcel.2017.08.019
Sitron CS, Brandman O (2020) Detection and degradation of stalled nascent chains via ribosome-associated quality control. Annu Rev Biochem 89:417–442. https://doi.org/10.1146/annurev-biochem-013118-110729
Slomnicki LP, Hallgren J, Vashishta A, Smith SC, Ellis SR, Hetman M (2018) Proapoptotic requirement of ribosomal protein l11 in ribosomal stress-challenged cortical neurons. Mol Neurobiol 55:538–553. https://doi.org/10.1007/s12035-016-0336-y
Sperling R, Henley D, Aisen PS, Raman R, Donohue MC, Ernstrom K, Rafii MS, Streffer J, Shi Y, Karcher K, Raghavan N, Tymofyeyev Y, Bogert J, Brashear HR, Novak G, Thipphawong J, Saad ZS, Kolb H, Rofael H, Sanga P, Romano G (2021) Findings of efficacy, safety, and biomarker outcomes of atabecestat in preclinical alzheimer disease: a truncated randomized phase 2b/3 clinical trial. Jama Neurol 78:293–301. https://doi.org/10.1001/jamaneurol.2020.4857
Squire LR (1992) Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99:195–231. https://doi.org/10.1037/0033-295x.99.2.195
Stein KC, Morales-Polanco F, van der Lienden J, Rainbolt TK, Frydman J (2022) Ageing exacerbates ribosome pausing to disrupt cotranslational proteostasis. Nature 601:637–642. https://doi.org/10.1038/s41586-021-04295-4
Sun MQ, Zhang MQ, Yuan Y, Yao C, Jing XQ (2018) Prostate Cancer: Expression and Clinical Significance of Ribosomal Protein L6. Prog Mod Biomed 18:745–749. https://doi.org/10.13241/j.cnki.pmb.2018.04.032
Suzuki M, Tezuka K, Handa T, Sato R, Takeuchi H, Takao M, Tano M, and Uchida Y (2022) Upregulation of ribosome complexes at the blood-brain barrier in alzheimer's disease patients. J Cereb Blood Flow Metab. 271678X221111602. https://doi.org/10.1177/0271678X221111602.
Tanzi RE, Bertram L (2005) Twenty years of the alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell 120:545–555. https://doi.org/10.1016/j.cell.2005.02.008
Tian JZ, Xie HG, Wang LN, Wang YH, Wang Hl, Shi J, Qin B, Fan DS, Ni JN, Sun YA the Guideline Panel of the Alzheimer's Disease Chinese (ADC) (2021) Chinese guideline for the diagnosis and treatment of Alzheimer's disease dementia(2020). Chin J Geriatr 40:269-283. https://doi.org/10.3760/cma.j.issn.0254-9026.2021.03.001
Turner RS, Hebron ML, Lawler A, Mundel EE, Yusuf N, Starr JN, Anjum M, Pagan F, Torres-Yaghi Y, Shi W, Mulki S, Ferrante D, Matar S, Liu X, Esposito G, Berkowitz F, Jiang X, Ahn J, Moussa C (2020) Nilotinib effects on safety, tolerability, and biomarkers in alzheimer’s disease. Ann Neurol 88:183–194. https://doi.org/10.1002/ana.25775
Ubaida-Mohien C, Lyashkov A, Gonzalez-Freire M, Tharakan R, Shardell M, Moaddel R, Semba RD, Chia CW, Gorospe M, Sen R, Ferrucci L (2019) Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. Elife 8. https://doi.org/10.7554/eLife.49874
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T (2023) Lecanemab in early alzheimer’s disease. N Engl J Med 388:9–21. https://doi.org/10.1056/NEJMoa2212948
Vasilopoulou CG, Sulek K, Brunner AD, Meitei NS, Schweiger-Hufnagel U, Meyer SW, Barsch A, Mann M, Meier F (2020) Trapped ion mobility spectrometry and pasef enable in-depth lipidomics from minimal sample amounts. Nat Commun 11:331. https://doi.org/10.1038/s41467-019-14044-x
Wang W, Nag S, Zhang X, Wang MH, Wang H, Zhou J, Zhang R (2015) Ribosomal proteins and human diseases: pathogenesis, molecular mechanisms, and therapeutic implications. Med Res Rev 35:225–285. https://doi.org/10.1002/med.21327
Wessels AM, Tariot PN, Zimmer JA, Selzler KJ, Bragg SM, Andersen SW, Landry J, Krull JH, Downing AM, Willis BA, Shcherbinin S, Mullen J, Barker P, Schumi J, Shering C, Matthews BR, Stern RA, Vellas B, Cohen S, MacSweeney E, Boada M, Sims JR (2020) Efficacy and safety of lanabecestat for treatment of early and mild alzheimer disease: the amaranth and daybreak-alz randomized clinical trials. Jama Neurol 77:199–209. https://doi.org/10.1001/jamaneurol.2019.3988
Wimo A, Jonsson L, Bond J, Prince M, Winblad B (2013) The worldwide economic impact of dementia 2010. Alzheimers Dement 9:1–11. https://doi.org/10.1016/j.jalz.2012.11.006
Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, Cedazo-Minguez A, Dubois B, Edvardsson D, Feldman H, Fratiglioni L, Frisoni GB, Gauthier S, Georges J, Graff C, Iqbal K, Jessen F, Johansson G, Jonsson L, Kivipelto M, Knapp M, Mangialasche F, Melis R, Nordberg A, Rikkert MO, Qiu C, Sakmar TP, Scheltens P, Schneider LS, Sperling R, Tjernberg LO, Waldemar G, Wimo A, Zetterberg H (2016) Defeating alzheimer’s disease and other dementias: a priority for european science and society. Lancet Neurol 15:455–532. https://doi.org/10.1016/S1474-4422(16)00062-4
Wu S, Tutuncuoglu B, Yan K, Brown H, Zhang Y, Tan D, Gamalinda M, Yuan Y, Li Z, Jakovljevic J, Ma C, Lei J, Dong MQ, Woolford JJ, Gao N (2016) Diverse roles of assembly factors revealed by structures of late nuclear pre-60s ribosomes. Nature 534:133–137. https://doi.org/10.1038/nature17942
Wu CC, Peterson A, Zinshteyn B, Regot S, Green R (2020) Ribosome collisions trigger general stress responses to regulate cell fate. Cell 182:404–416. https://doi.org/10.1016/j.cell.2020.06.006
Wu T, Lin D, Cheng Y, Jiang S, Riaz MW, Fu N, Mou C, Ye M, Zheng Y (2022) Amyloid cascade hypothesis for the treatment of alzheimer’s disease: progress and challenges. Aging Dis 13:1745–1758. https://doi.org/10.14336/AD.2022.0412
Xing M, Mao JJ, Chen WL, Li ZF (2020) Analyses of magnetic resonance spectroscopy and ultrastructure changes in the hippocampus of APP/PS1 double transgenic AD model mice. Acta Lab Anim Sci Sin 28:236–241. https://doi.org/10.3969/j.issn.1005-4847.2020.02.013
Xiong XY, Mei H, Ye NL, Xiao F, Lu ZY (2020) 3.0 T MRI determination of hippocampus volume, olfactory bulb volume and cognitive impairment in Alzheimer’s disease patients. Chin J Magn Reson Imaging 11:858–861. https://doi.org/10.12015/issn.1674-8034.2020.10.005
Yang C, Zang W, Ji Y, Li T, Yang Y, Zheng X (2019a) Ribosomal protein l6 (rpl6) is recruited to dna damage sites in a poly(adp-ribose) polymerase-dependent manner and regulates the dna damage response. J Biol Chem 294:2827–2838. https://doi.org/10.1074/jbc.RA118.007009
Yang T, Dang Y, Ostaszewski B, Mengel D, Steffen V, Rabe C, Bittner T, Walsh DM, Selkoe DJ (2019b) Target engagement in an alzheimer trial: crenezumab lowers amyloid beta oligomers in cerebrospinal fluid. Ann Neurol 86:215–224. https://doi.org/10.1002/ana.25513
Yang G, Pei YN, Shao SJ, Gao YS, Zhang SJ, Hu C, Feng SN, Xue WG (2020) Effects of electroacupuncture at “Baihui” and “Yongquan” on the levels of synaptic plasticity related proteins postsynaptic density-95 and synaptophysin in hippocampus of APP/PS1 mice. Acupunct Res 45:310–314. https://doi.org/10.13702/j.1000-0607.190012
Yin F, Gong SQ, Li FZ, Wang SS, Liu Y, Cheng SW (2018) Current Status of Genetic Engineering Mouse Models of Alzheimer’s Disease. Med Recapitulate 24:241–247. https://doi.org/10.3969/j.issn.1006-2084.2018.02.007
Yu CH, Dang Y, Zhou Z, Wu C, Zhao F, Sachs MS, Liu Y (2015) Codon usage influences the local rate of translation elongation to regulate co-translational protein folding. Mol Cell 59:744–754. https://doi.org/10.1016/j.molcel.2015.07.018
Zhang L, Wang Y, Xiayu X, Shi C, Chen W, Song N, Fu X, Zhou R, Xu YF, Huang L, Zhu H, Han Y, Qin C (2017) Altered gut microbiota in a mouse model of alzheimer’s disease. J Alzheimers Dis 60:1241–1257. https://doi.org/10.3233/JAD-170020
Zhang XL, Zeng JY, Chen X, Yuan G (2021) Comparison and Interpretation of Chinese and British Guidelines about Therapeutic Drugs for Alzheimer’s Disease. Chin Gen Pract 24:1454–1458. https://doi.org/10.12114/j.issn.1007-9572.2021.00.121
Zhao K, Ding YH, Han Y, Fan Y, Aaron FA, Han T, Jin D, Liu B, Lu J, Song CY, Wang P, Wang DW, Wang Q, Xu KB, Yang HW, Yao HX, Zheng YJ, Yu CS, Zhou B, Zhang XQ, Zhou YY, Jiang TZ, Zhang X, Liu Y (2020) Independent and reproducible hippocampal radiomic biomarkers for multisite Alzheimer’s disease: diagnosis, longitudinal progress and biological basis. Sci Bull 65:1103–1113. https://doi.org/10.1016/j.scib.2020.04.003
Zhou X, Liao WJ, Liao JM, Liao P, Lu H (2015) Ribosomal proteins: functions beyond the ribosome. J Mol Cell Biol 7:92–104. https://doi.org/10.1093/jmcb/mjv014
Zhou J, Heo HY, Knutsson L, van Zijl P, Jiang S (2019) Apt-weighted mri: techniques, current neuro applications, and challenging issues. J Magn Reson Imaging 50:347–364. https://doi.org/10.1002/jmri.26645
Funding
This study was supported by the Natural Science Foundation of Shandong (ZR2023QH159); the Education Department of Jilin Province ‘13th Five-Year’ Science and Technology Project (JJKH20200895KJ); the Science and Technology Ability Promotion Plan of Health Commission of Jilin Province (2019J058); the Jilin Provincial Science and Technology Department Key Research and Development-Medicine and Health Field (20200404065YY); and Traditional Chinese Medicine science and technology project of Shandong Province (Z-2023042).
Author information
Authors and Affiliations
Contributions
MQ, GC and JS designed the study. LN drafted the manuscript and edited and revised it critically for important intellectual content. LN and GJ contributed to the quality assessment, data analysis, and interpretation of the data. XT and QL carefully revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval
All experimental protocols were approved by the Animal Experiment Ethics Committee of Changchun University of Chinese Medicine, ensuring compliance with the principles of animal protection, animal welfare, and ethics (N0. 2022648).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, L., Wang, G., Song, Q. et al. Proteomics revealed an association between ribosome-associated proteins and amyloid beta deposition in Alzheimer's disease. Metab Brain Dis 39, 263–282 (2024). https://doi.org/10.1007/s11011-023-01330-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-023-01330-3