Abstract
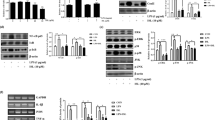
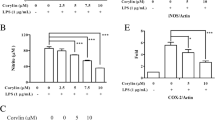
Microglia are the tissue-resident immune cells of the central nervous system. As a part of the innate immune response, NLR Family Pyrin Domain Containing Protein 3 (NLRP3) inflammasome activation leads to cleavage of caspase-1 and triggers secretion of proinflammatory cytokines and may also result in pyroptotic cell death. Inflammasome activation plays a crucial role in inflammatory conditions; aberrant activation of inflammasome contributes to the pathogenesis of neurodegenerative diseases. Diethyl Maleate (DEM) is a promising antiinflammatory chemical to alleviate inflammasome activation. In this study, NLRP3 inflammasome was activated in N9 murine microglia via 1 µg/ml LPS (Lipopolysaccharide) for 4 h and 5 mM ATP (Adenosine 5′-triphosphate) for 1 h, respectively. We demonstrated that 1 h pretreatment of DEM attenuated NLRP3 inflammasome activation in microglial cells. Besides, mitochondrial ROS decreased upon DEM pretreatment in inflammasome-induced cells. Likewise, it ameliorated pyroptotic cell death in microglia. DEM is a potent activator of Nrf2 transcription factor, the key regulator of the antioxidant response pathway. Nrf2 has been a significant target to decrease aberrant inflammasome activation through the antioxidant compounds, including DEM. Here, we have shown that DEM increased Nrf2 translocation to the nucleus, resulting in Nrf2 target gene expression in microglia. In conclusion, DEM is a promising protective agent against NLRP3 inflammasome activation.




Similar content being viewed by others
Abbreviations
- ARE:
-
Antioxidant response element
- ATP:
-
Adenosine 5′-triphosphate
- ASC:
-
Apoptosis-associated speck-like protein containing a CARD
- bZIP:
-
Basic-region leucine zipper
- CNC:
-
Cap “n” collar
- CNS:
-
Central nervous system
- DEM:
-
Diethyl Maleate
- Gclc:
-
Glutamate cysteine ligase catalytic subunit
- Gclm:
-
Glutamate cysteine ligase regulatory subunit
- HO-1:
-
Heme oxygenase-1
- Keap1:
-
Kelch-like ECH-associated protein 1
- LPS:
-
Lipopolysaccharide
- NF-κB:
-
Nuclear factor-κB
- NLRP3:
-
NLR family pyrin domain containing protein 3
- Nqo1:
-
NAD(P)H:quinone oxidoreductase 1
- Nrf2:
-
Nuclear factor-erythroid-2-related factor 2
- PRR:
-
Pattern recognition receptor
- ROS:
-
Reactive oxygen species
References
Ahmed SM, Luo L, Namani A, Wang XJ (1863) Tang X (2017) Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis 2:585–597
Cuadrado A, Martin-Moldes Z, Ye J, Lastres-Becker I (2014) Transcription factors NRF2 and NF-kappaB are coordinated effectors of the rho family, GTP-binding protein RAC1 during inflammation. J Biol Chem 289:15244–15258
Dubbelaar ML, Kracht L, Eggen BJL, Boddeke E (2018) The kaleidoscope of microglial phenotypes. Front Immunol 9:1753
Guo H, Callaway JB, Ting JP (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21:677–687
Haque ME, Akther M, Jakaria M, Kim IS, Azam S, Choi DK (2020) Targeting the microglial NLRP3 inflammasome and its role in Parkinson’s disease. Mov Disord 35:20–33
Harada N, Kanayama M, Maruyama A, Yoshida A, Tazumi K, Hosoya T, Mimura J, Toki T, Maher JM, Yamamoto M, Itoh K (2011) Nrf2 regulates ferroportin 1-mediated iron efflux and counteracts lipopolysaccharide-induced ferroportin 1 mRNA suppression in macrophages. Arch Biochem Biophys 508:101–109
He Y, Hara H, Nunez G (2016) Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 41:1012–1021
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678
Hennig P, Garstkiewicz M, Grossi S, Di Filippo M, French LE, Beer HD (2018) The crosstalk between Nrf2 and inflammasomes. Int J Mol Sci 19:562
Holbrook JA, Jarosz-Griffiths HH, Caseley E, Lara-Reyna S, Poulter JA, Williams-Gray CH, Peckham D, McDermott MF (2021) Neurodegenerative disease and the NLRP3 inflammasome. Front Pharmacol 12:643254
Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M (2000) Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 275:16023–16029
Iso T, Suzuki T, Baird L, Yamamoto M (2016) Absolute amounts and status of the Nrf2-Keap1-Cul3 complex within cells. Mol Cell Biol 36:3100–3112
Jo EK, Kim JK, Shin DM, Sasakawa C (2016) Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13:148–159
Jones JJ, Fan J, Nathens AB, Kapus A, Shekhman M, Marshall JC, Parodo J, Rotstein OD (1999) Redox manipulation using the thiol-oxidizing agent diethyl maleate prevents hepatocellular necrosis and apoptosis in a rodent endotoxemia model. Hepatology 30:714–724
Kang KW, Pak YM, Kim ND (1999) Diethylmaleate and buthionine sulfoximine, glutathione-depleting agents, differentially inhibit expression of inducible nitric oxide synthase in endotoxemic mice. Nitric Oxide 3:265–271
Kano SI, Choi EY, Dohi E, Agarwal S, Chang DJ, Wilson AM, Lo BD, Rose IVL, Gonzalez S, Imai T, Sawa A (2019) Glutathione S-transferases promote proinflammatory astrocyte-microglia communication during brain inflammation. Sci Signal 12:2124
Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M (2016) Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 7:11624
Krafczyk N, Klotz LO (2022) FOXO transcription factors in antioxidant defense. IUBMB Life 74:53–61
Mamik MK, Power C (2017) Inflammasomes in neurological diseases: emerging pathogenic and therapeutic concepts. Brain 140:2273–2285
Man SM, Kanneganti TD (2015) Regulation of inflammasome activation. Immunol Rev 265:6–21
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E (2018) Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 17:588–606
Nathens AB, Marshall JC, Watson RW, Dackiw AP, Rotstein OD (1996) Diethylmaleate attenuates endotoxin-induced lung injury. Surgery 120:360–366
Prinz M, Jung S, Priller J (2019) Microglia biology: one century of evolving concepts. Cell 179:292–311
Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, Donini SD, Ricciardi-Castagnoli P (1989) Monokine production by microglial cell clones. Eur J Immunol 19:1443–1448
Robertson H, Dinkova-Kostova AT, Hayes JD (2020) NRF2 and the ambiguous consequences of its activation during initiation and the subsequent stages of tumourigenesis. Cancers (Basel) 12:3609
Sarkar S, Malovic E, Harishchandra DS, Ghaisas S, Panicker N, Charli A, Palanisamy BN, Rokad D, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG (2017) Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson’s disease. NPJ Parkinsons Dis 3:30
Satoh T, Trudler D, Oh CK, Lipton SA (2022) Potential therapeutic use of the rosemary diterpene carnosic acid for Alzheimer’s disease, Parkinson’s disease, and long-COVID through NRF2 activation to counteract the NLRP3 inflammasome. Antioxidants. https://doi.org/10.3390/antiox11010124
Silva-Islas CA, Maldonado PD (2018) Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol Res 134:92–99
Song N, Li T (2018) Regulation of NLRP3 inflammasome by phosphorylation. Front Immunol 9:2305
Stansley B, Post J, Hensley K (2012) A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. J Neuroinflam 9:115
Swanson KV, Deng M, Ting JP (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19:477–489
Taguchi K, Yamamoto M (2020) The KEAP1-NRF2 system as a molecular target of cancer treatment. Cancers. https://doi.org/10.3390/cancers13010046
Timmerman R, Burm SM, Bajramovic JJ (2018) An overview of in vitro methods to study microglia. Front Cell Neurosci 12:242
Tonelli C, Chio IIC, Tuveson DA (2018) Transcriptional regul Nrf2. Antioxid Redox Signal 29:1727–1745
Tufekci KU, Ercan I, Isci KB, Olcum M, Tastan B, Gonul CP, Genc K, Genc S (2021) Sulforaphane inhibits NLRP3 inflammasome activation in microglia through Nrf2-mediated miRNA alteration. Immunol Lett 233:20–30
Ulasov AV, Rosenkranz AA, Georgiev GP, Sobolev AS (2022) Nrf2/Keap1/ARE signaling: towards specific regulation. Life Sci 291:120111
Voet S, Srinivasan S, Lamkanfi M, van Loo G (2019) Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol Med 11:e10248
Yu JW, Lee MS (2016) Mitochondria and the NLRP3 inflammasome: physiological and pathological relevance. Arch Pharm Res 39:1503–1518
Zhang C, Zhao M, Wang B, Su Z, Guo B, Qin L, Zhang W, Zheng R (2021) The Nrf2-NLRP3-caspase-1 axis mediates the neuroprotective effects of celastrol in Parkinson’s disease. Redox Biol 47:102134
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469:221–225
Funding
The authors declare that no funds or grants were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
CK and CPG conceived the study, performed the experiments. CK analyzed and interpreted the data and wrote the manuscript. SG conceived the study, interpreted the data, and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Research involving human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kiser, C., Gonul, C.P. & Genc, S. Nrf2 activator Diethyl Maleate attenuates ROS mediated NLRP3 inflammasome activation in murine microglia. Cytotechnology 76, 197–208 (2024). https://doi.org/10.1007/s10616-023-00609-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-023-00609-8