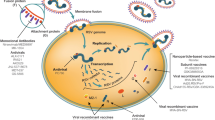
Abstract
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infection (LRTI) in children, and is associated with long-term pulmonary sequelae for up to 30 years after infection. The mainstay of RSV management is supportive therapy such as supplemental oxygen. Palivizumab (Synagis™–AstraZeneca), a monoclonal antibody targeting the RSV F protein site II, has been licensed for the prevention of RSV in high-risk groups since 1998. There has been recent promising progress in preventative strategies that include vaccines and long-acting, high-potency monoclonal antibodies. Nirsevimab (Beyfortus™–AstraZeneca/Sanofi), a monoclonal antibody with an extended half-life, has recently been registered in the European Union and granted licensure by the US Food and Drug Administration. Furthermore, a pre-fusion sub-unit protein vaccine has been granted licensure for pregnant women, aimed at protecting their young infants, following established safety and efficacy in clinical trials (Abrysvo™–Pfizer). Also, multiple novel antiviral therapeutic options are in early phase clinical trials. The next few years have the potential to change the landscape of LRTI through improvements in the prevention and management of RSV LRTI. Here, we discuss these new approaches, current research, and clinical trials in novel therapeutics, monoclonal antibodies, and vaccines against RSV infection in infants and children.
Similar content being viewed by others
Respiratory syncytial virus (RSV) is the most common cause of respiratory tract infections in children. |
There are currently only two medications licensed for the prevention of RSV infection in infants: palivizumab (Synagis™–AstraZeneca) and nirsevimab (Beyfortus™–AstraZeneca/Sanofi). |
Steady progress is being made with regard to new potential vaccine candidates targeting vaccination of pregnant woman and their infants, with the recent licensing of the first maternal vaccine against RSV (Abrysvo™–Pfizer). |
1 Introduction
Lower respiratory tract infections (LRTIs) are the leading cause of death in children under 5 years outside the neonatal period, accounting for approximately 12% of global deaths in children [1, 2]. Most LRTIs in young children are caused by viruses (60%), of which respiratory syncytial virus (RSV) accounts for the largest portion (31%) [3,4,5]. RSV is a single-stranded, negative-sense ribonucleic acid (RNA) virus, which is enveloped by a host plasma membrane-derived lipid bilayer. The ten-gene RSV genome encodes for 11 proteins, the two most important ones being transmembrane glycoproteins: attachment protein (G protein) and fusion protein (F protein) [6, 7]. The binding of RSV to the respiratory epithelial cell is enabled by the G protein [8,9,10], while the F protein mediates fusion between viral and cell membranes, thereby permitting viral penetration into the cell [11]. The F protein possesses two unique forms: before binding to the host cell, a stable pre-fusion conformation, and after binding, a highly stable post-fusion conformation [11, 12].
The range of infections caused by RSV extends across the whole spectrum of respiratory tract infections from asymptomatic upper respiratory tract infection to severe LRTI, and death [13, 14]. The most serious common presentation of RSV infection is bronchiolitis and pneumonia [13]. Most children are infected with RSV during the first year of life, and virtually all have been infected at least once by 2 years of age [15, 16]. RSV most commonly infects term infants, and the severity of the resulting disease is determined through the intensity of the initial infection, such as the initial viral load, and the response of the host to the infection [17, 18]. Specific underlying medical conditions predispose the infant to an increased risk of severe disease; these include prematurity, congenital cardiac disease, and bronchopulmonary dysplasia (BPD) [18,19,20,21,22,23].
In 2019, there were approximately 33.0 million RSV-associated acute LRTIs, 3.6 million RSV-associated acute LRTI hospital admissions, and 66,000–190,000 RSV-attributable deaths in children aged less than 60 months. More than 95% of RSV-associated deaths occur in low- and middle-income countries (LMICs), and 50% of deaths occur in infants less than 6 months of age [24, 25]. RSV LRTI during infancy has been reported to lead to long-term pulmonary sequelae, with multiple studies reporting an increase in recurrent wheezing episodes for at least the first decade after infection, and non-bronchodilator responsive obstructive lung disease [26,27,28,29,30,31,32,33,34,35,36].
The mechanism of transmission of RSV is droplet spread, either via airborne particles or contact with contaminated surfaces. Inoculation occurs through the nasopharyngeal mucosa or conjunctival membranes [37], with an incubation period of 3–5 days, followed by viral spread to the smaller airways [37,38,39,40], with subsequent destruction of the epithelium and loss of ciliary motion, as well as indirect host immune response effects [41].
2 Immunology of RSV
An understanding of the immunology involved during an RSV infection is imperative in understanding the host’s response to an RSV infection, the mechanisms that RSV employ to evade the host’s immune system, and the potential targets for prevention and treatment of RSV infection.
Cytotoxic T cell immune responses are essential for the resolution of RSV infection. CD4+ T cells stimulate B cell antibody production, and CD8+ T cells are cytopathic to RSV-infected cells [42]. Acquisition of specific immunological memory, including humoral immune responses, results in neutralizing antibodies and production of RSV-specific cytotoxic T cells, resulting in a lower likelihood of severe disease following subsequent RSV infections [43].
Serum neutralizing antibodies against RSV are associated with a reduced risk of RSV infection progressing to LRTI. However, neutralizing antibodies acquired through natural RSV infection are generally transient [44, 45]. Maternal acquired RSV antibodies, gained through transplacental and breastmilk transfer, confer some protection, with a lower risk of RSV during early infancy; however, this is also transient [46,47,48]. Transplacental antibody transfer is less efficient during the early stages of pregnancy, and this contributes greatly to the severity of infection experienced in prematurely born children [49, 50].
RSV employs multiple mechanisms to decrease the effectiveness of the host’s immune response: NS1+NS2 (non-structural proteins) inhibition of interferon responses [51], interference in the Toll-like receptor (TLR) signaling pathway through the binding of protein F to TLR 4 [52], and CX3CR1 (fractalkine) binding by secretory protein G binding, thereby altering chemotaxis, as well as acting as a decoy for antibody binding [8, 53].
Six main antigenic epitopes are found on the RSV F protein surface (Ø and I–V). Sites Ø, III, and V are only exposed during the pre-fusion F protein conformation, while I, II, and IV are exposed on both the pre- and post-fusion F protein conformations [11, 12, 54]. The F protein is the main target for interventions such as vaccines and monoclonal antibodies. This is due to the number of exposed surface epitopes, its requirement for cell penetration, and the fact that it is highly genetically conserved. Epitopes II and IV are the main neutralizing epitopes, and whilst they do not prevent viral attachment to the affected cells, they effectively block fusion of the viral and host cellular membranes [54]. The G protein is a less efficient neutralization antigen than the F protein.
Treatment of RSV infection is largely symptomatic, with few specific treatment options available. Therefore, the management of RSV has focused mainly on prevention of the disease, through two approaches: passive and active immunization. The purpose of this review is to give an update on progress made in the prevention and treatment of RSV infection in children.
3 Passive Immunization
Passive immunization involves administration of antibodies targeting a specific pathogen, and is used in instances where immediate protection is required or where timeous production of antibodies is not possible, such as in newborns and during early infancy. Prevention of RSV through passive immunization involves administration of polyclonal or monoclonal RSV-neutralizing antibodies (Table 1).
The first commercially available preparation was RSV immune globulin intravenous (RSV-IGIV) (RespiGam™) in 1992. This preparation contained purified polyclonal antibodies from donors with high-titer RSV neutralizing activity [55]. Administered monthly to at-risk children under 2 years, RSV-IGIV resulted in a decreased risk of RSV LRTI, severe RSV LRTI, and RSV hospitalization and decreased the duration of RSV-associated intensive care unit (ICU) admission [56, 57]. RSV-IGIV did result in an increase in hypersensitivity reactions, but not more than was reported for standard intravenous immunoglobulin preparations [58].
Palivizumab (Synagis™–AstraZeneca) is a humanized monoclonal antibody directed at RSV protein F site II epitope, and until recently, it was the only licensed treatment for the prevention of RSV LRTI [59]. In a randomized, double-blind, placebo-controlled trial in high-income countries (HICs) (IMpact-RSV), children at high risk for severe RSV LRTI (prematurity or BPD) received either monthly palivizumab or placebo for 5 months [60]. Children receiving palivizumab with prematurity and no BPD had a 78% risk reduction for hospitalization, whilst a 39% risk reduction was shown for children with prematurity and BPD.
The high cost of palivizumab restricts its use, even in HICs such as the USA, where the cost runs to approximately $1700–12,500 per RSV season [61,62,63]. This has led to specific restrictive recommendations being applied even in those countries and regions where it is available, such as the USA, Europe, and Australasia [64,65,66].
The development of next-generation, single-dose, long-acting monoclonal antibodies was facilitated through the initial introduction of a triple YTE mutation (M252Y/S254T/T256E) into the CH2 domain of the Fc portion of MEDI-524 (IgG1), resulting in motavizumab (Numax™–MedImmune, Inc.) [67, 68]. This addition enhances Fc receptor binding, with a subsequent increase of up to four times the half-life, with this increase not being due to interactions with serum components, as well as an increase in serum levels, not due to alteration in distribution. Importantly, the YTE mutation resulted in no discernable structural changes to the IgG molecule, nor any functional impairment. Motavizumab provides 20 times higher in vitro neutralization activity, due to it having 70 times the affinity for the RSV F protein compared with palivizumab [69]. Motavizumab was non-inferior to palivizumab in a large, phase 3, randomized, double-blind study comparing outcomes in children born before 36 weeks gestation, either younger than 6 months or younger than 2 years with chronic lung disease. Motavizumab was associated with a relative risk reduction of 26% and 50% for RSV hospitalization and acute, medically attended (MA) LRTI (MALRTI), respectively [70]. However, due to an increase in cutaneous hypersensitivity reactions in recipients, motavizumab was not granted licensure by the US Food and Drug Administration (FDA), and further development has been discontinued [71].
Suptavumab (Regeneron Pharmaceuticals, Inc.), a long-acting monoclonal antibody with affinity for the F protein pre-fusion site IV epitope, was discontinued after failing to show protection against RSV-associated hospitalization or outpatient illness in a phase 3 efficacy trial in healthy preterm infants less than 6 months of age [72]. This failure was likely due to a novel genetic strain of RSV B harboring target epitope site mutations.
Nirsevimab (Beyfortus™–AstraZeneca/Sanofi), a recombinant human IgG1 monoclonal antibody that targets the highly conserved site Ø of the pre-fusion RSV F protein, can be administered as a single dose prior to the RSV season, intramuscularly [73]. Nirsevimab inhibits the fusion of the RSV and the respiratory epithelium, thereby inhibiting viral entry into the cell, and is equally effective against both RSV A and RSV B strains. In a phase 2b, randomized, placebo-controlled trial in healthy premature infants (< 37 weeks gestation), a single dose of nirsevimab was reported to have a 70% and 78% vaccine efficacy (VE) against RSV MALRTI and RSV LRTI hospitalization [74]. Furthermore, in late preterm (> 34 weeks gestation) and term infants, the VE was 74% and 62% against RSV LRTI and hospitalization for RSV LRTI, respectively, through to 180 days post-enrolment [75]. Nirsevimab also continued to confer protection through into the second RSV season, as indicated by a 43% lower risk of RSV MALRTI in the treatment group between 361 and 511 days [76]. In a pooled analysis of the preterm and term infant data mentioned above, nirsevimab resulted in a 80% relative risk reduction for RSV MALRTI [77]. Nirsevimab was registered for use in the European Union and the United Kingdom in November 2022 and granted licensure by the US FDA in July 2023, and was recommended by the Centre for Disease Control and Prevention Advisory Committee on Immunization Practices and the American Academy of Pediatrics in August 2023, providing the first additional option for the prevention of RSV LRTI in over 20 years. Possible side effects are the development of a rash and local effects at the site of the injection, as well as hypersensitivity reaction, whereas contra-indications include administering the drug to children that have any history of serious hypersensitivity reactions to the active ingredients in the preparation.
A further long-acting monoclonal antibody with affinity for epitope site IV of the RSV F protein, clesrovimab (Merck Sharp & Dohme), is being evaluated in a multicenter, randomized, partially blinded, phase 3 trial, with estimated completion in April 2026, after a phase 2a study reported a VE of 74% for prevention of MALRTI in infants during the first 6 months of life. Safety and efficacy will also be compared relative to palivizumab in infants and children at increased risk of severe RSV disease (NCT04938830 + NCT04767373).
4 Active Immunization
The initial 1966 failure of a formalin inactivated whole virus RSV vaccine (FIRSV) resulted in a decades-long delay in RSV vaccine development [78]. Children receiving the vaccine were provided no protection and developed more severe disease following RSV infection in the following season, including two deaths and 80% hospitalization in cases, especially if sero-negative prior to vaccination.
The unfavorable outcome of the vaccine was attributed to vaccine-associated enhanced disease (VAED) due to a T-helper cell 2 type dominant immune response and induction of non-neutralizing antibodies. Neutralizing antibodies are the desired effect of vaccines against viral pathogens [79]. They bind to receptor binding domains of viruses and inhibit viral attachment, entry, and fusion with the host cell. They furthermore induce the formation of immune complexes, which triggers further enhancement of the immune response. An abnormal accumulation of these immune complexes may cause overstimulation of the immune response, and eventual VAED [79, 80].
There are numerous RSV vaccine candidates in various developmental stages, targeting different groups, including infants, pregnant women, and the elderly (see Table 2 and Supplementary Table 1 in the electronic supplementary material). The aim of maternal vaccination, with the subsequent maternal–fetal antibody transfer, is to prevent RSV LRTI in young infants, when 50% of RSV-associated hospitalizations and deaths occur [81,82,83]. The effectiveness of vaccinating pregnant woman as a strategy to prevent respiratory infections during infancy has been illustrated by the administration of inactivated influenza vaccine (IIV) and acellular pertussis vaccine to pregnant women [84, 85]. Infants born to women vaccinated with IIV were less likely to become infected with influenza during the first 6 months of life (VE 29%) and were 43% less likely to be hospitalized for all-cause pneumonia during the first 3 months of life [86, 87], while maternal acellular pertussis vaccination protected children for the age period of greatest mortality caused by pertussis, namely the first 2 months of life.
Numerous RSV vaccine candidates are currently being investigated [81]. For an updated snapshot, visit PATH at https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/. Different mechanisms for stimulating the host immune response to provide a future protective response against RSV are being examined. These include live attenuated virus vaccines (LAV), chimeric vaccines, protein-based vaccines (sub-unit or particle, including nanoparticles), nucleic acid vaccines (NAV), and recombinant vector-based vaccines.
LAV contain live replicating pathogens with reduced virulence that elicit either a robust cellular of humoral immune respone, which in the past have been either too reactogenic or not reactogenic enough, highlighting the difficulties for this type of vaccine [88]. The intranasal route of LAV administration results in stronger mucosal immunity than systemic administered vaccines. Current RSV LAV include pathogens attenuated through reverse genetic engineering with deletion of proteins that regulate viral synthesis or responses [89]. Most LAV candidates are still undergoing phase 1 or phase 2 trials [90], including VAD00001 (SP0125), undergoing a safety, immunogenicity, and dosing, randomized, placebo-controlled trial in children 6–18 months.
Chimeric vaccines are hybrid organisms comprising selected attenuated viruses with genetic material from the organism of interest. There are three candidates currently in phase 1 trials: parainfluenza 5 virus/RSV chimera, Bacillus Calmette-Guerin expressing RSV N gene, and Sendai virus/RSV protein F [90].
Protein-based vaccines can be particle or sub-unit based and elicit a robust humoral and cellular immune response. They contain nanoscopic particles that mimic selected antigens on the viral surface. The F protein, both pre-fusion and post-fusion conformations, has been the main antigenic protein utilized, but G protein, small hydrophobic protein, matrix protein, and nucleocapsid (N) protein have also been investigated. There are two maternal F protein vaccines currently in phase 3 trials, with the Abrysvo™ (Pfizer), becoming the first maternal vaccine receiving FDA approval (Table 2). The F protein vaccine, albeit in early phase trials, is also being evaluated in the pediatric population, and two F protein vaccines in adults older than 65 years of age have recently become the first RSV vaccines to be licensed [91, 92].
In a phase 3, randomized, observer-blind, placebo-controlled trial, a pre-fusion RSV F nanoparticle vaccine (Novavax) administered to healthy pregnant women between 28 and 36 weeks gestational age did not meet predetermined efficacy targets [83]. A single dose of vaccine was associated with a 39% reduction of the primary endpoint of RSV-associated MALRTI in the first 3 months of life in the infants, with a VE of 40.5% in LMICs compared to 37% in HICs.
A further phase 3, randomized, double-blind, placebo-controlled study, evaluating the VE of a single dose of intramuscular pre-fusion RSV F protein vaccine (RSVPreF3–GlaxoSmithKline) administered to pregnant women 18–49 years of age for protecting against RSV-associated LRTIs in their infants (NCT04605159), was voluntarily halted pending safety data analysis, due to an excess of premature deliveries and associated higher infant mortality rate in the vaccine arm [93]. Safety data presented recently (unpublished data) reported that RSVPreF3-Mat had an acceptable reactogenicity profile in maternal participants and that no imbalances were observed in pregnancy outcomes between groups, including fetal deaths. Preterm births were considered an identified risk for pregnant women following RSVPreF3-Mat vaccination, which led to the discontinuation of RSVPreF3-Mat development.
A phase 2, observer-blind, placebo-controlled trial of an RSV pre-fusion maternal vaccine administered to 213 pregnant women 18–40 years old during the second or third trimester found the vaccine to be well tolerated, with successful induction and transfer of maternal neutralizing antibodies against both RSV A + B to the newborn (NCT04126213) [94]. A further phase 2b trial, evaluating the efficacy of a bivalent maternal RSV A and RSV B stabilized pre-fusion protein vaccine administered to women between 24 and 36 weeks gestational age, reported robust neutralizing antibody responses in the pregnant women, with highly efficient transplacental antibody transfer to the newborn (NCT04032093). Neutralizing antibodies in the infant persisted above the protective threshold against RSV LRTI through to 180 days of age. The MATISSE trial (NCT04424316) further reported a VE against severe RSV MALRTI during the first 90 days of life at 81.8% (99.5% confidence interval [CI] 40.6–96.3) and 69.4% (97.58% CI 44.3–84.1) through to 180 days of life [95, 96]. These results led the US FDA to grant licensure in August 2023, the first vaccine licensed for maternal vaccination (Abrysvo™–Pfizer). Although, after sub-group analysis of live birth outcomes by country income status, there was no difference between HICs and LMICs with regard to increase in incidence of preterm birth, an increase in preterm births was noted in vaccine recipients (8.3%) compared with placebo recipients (4.0%) in South Africa (upper middle-income country) [97].
NAV such as messenger RNA (mRNA) vaccines were successfully utilized for coronavirus disease 2019 (COVID-19) vaccines [98]. Laboratory-based, pre-fabricated mRNA is utilized to stimulate the recipient’s cellular nucleus to encode for the production of a protein or part thereof [99]. This stimulates the host immune system to produce specific antibodies. An mRNA-1345 vaccine (Moderna), which encodes stabilized RSV pre-fusion protein, is being evaluated in women of child-bearing age (18–40 years) and RSV sero-positive children aged 12–59 months, with results expected by September 2023 (NCT04528719).
Replicating or non-replicating viruses with extra genetic material from a pathogen of interest make up recombinant vector vaccines, with this engineered genetic material delivered to the recipient, eliciting an immune response. An adenovirus vector vaccine, Ad26.RSV.pre-F, is currently undergoing a phase 2 trial in RSV sero-positive toddlers, evaluating its safety, tolerability, and immunogenicity. Enrolment has been completed.
5 Treatment of RSV infection
The management of RSV LRTI is mainly supportive, with oxygen therapy and nutritional support. Nebulized hypertonic saline, beta2-agonists, nebulized adrenalin, nebulized ipratropium bromide, montelukast, and corticosteroids have been shown to be largely ineffective and are not recommended [100,101,102,103,104,105,106,107,108,109,110,111].
The development of a satisfactory antiviral medication against RSV infection remains elusive; however, steady progress is being made, with many potential candidate molecules undergoing clinical trials. One of the difficulties of RSV treatment is that medications need to be administered very early in the disease process, due to the RSV inoculation time being 3–5 days and severe disease generally developing within 4 days of symptom onset, a very small window of opportunity. There are no biomarkers available to indicate which children might develop severe disease, and the majority will not develop severe disease, thereby not necessitating treatment.
The most researched antiviral drug targeting RSV is ribavirin, a broad-spectrum antiviral guanosine analog that inhibits the inosine monophosphate dehydrogenase enzyme, leading to decreased levels of guanosine triphosphate, which is needed by RSV for replication [112, 113]. Although ribavirin is available in both oral and intravenous preparations, its use has been hampered by its high cost, poor tolerability, and many reported side effects. In a recent systematic review, no difference in mortality rate was shown between individuals treated for RSV LRTI with ribavirin and those receiving supportive care, except for a lower mortality in individuals with hematological disease [114]. Furthermore, studies comparing oral and aerosolized preparations in adult recipients of hematopoietic cell and lung transplants have shown similar outcomes and safety profiles [112, 115,116,117].
Novel drug therapies being investigated include an F protein binding nanobody (ALX-0171 [Gontivimab–Ablynx], fusion inhibitors (GS-5806 [presatovir–Gilead Sciences], JNJ-53718678 [rilematovir–Janssen Pharmaceutical], BTA-C585 [enzaplatovir–Aviragen Therapeutics], and AK-0529 [ziresovir–Ark Biopharmaceutical]), a non-fusion N protein inhibitor (EDP-938 [Mavyret–Enanta Pharmaceuticals]), and an RSV polymerase inhibitor (ALS-008176 [lumicitabine–Alios BioPharma/Janssen Pharmaceuticals]) [118,119,120,121,122].
ALX-0171 is a trimeric nanobody that binds the F protein epitope site II [123]. Nanobodies are the smallest available heavy-chain portion of an immunoglobulin that retains its function and lends itself to aerosol delivery [124]. However, in a double-blind, placebo-controlled, phase 2b trial evaluating the safety and antiviral properties of nebulized ALX-0171 in 175 children hospitalized with RSV LRTI, no difference in clinical outcomes, time to clinical response, or global severity score was shown [125].
Fusion inhibitors inhibit the fusion of RSV F protein with the cells of the respiratory tract and act on a late-stage fusion intermediate during the process of the RSV F protein conformational change [126]. In a phase 2b, double-blind, placebo-controlled, adult RSV, challenge study, presatovir reduced viral load and severity of the clinical illness in 54 cases [119]. However, in another phase 2b, double-blind, placebo-controlled trial, in 60 hematopoietic cell transplant recipients with RSV LRTI, presatovir did not improve virological or clinical outcomes [119, 127]. JNJ-53718678 was well tolerated and exhibited antiviral activity in a phase 1b trial in children 1–24 months of age, and caused a reduction in viral load and clinical disease severity in healthy adults in a challenge study [121, 128]. Further studies examining the efficacy of this compound will be forthcoming. Enzaplatovir is undergoing a phase 2a, double-blind, placebo-controlled, challenge study in healthy adult volunteers, evaluating its safety and antiviral activity (NCT02718937). In a phase 2 trial, AK-0529 was reported to be safe and to reduce the viral load in children (1–24 months) infected with RSV, and a phase 3, randomized, double-blind, placebo-controlled trial in infants hospitalized with RSV infection is underway (NCT04231968).
In a phase 2, randomized, double-blind, placebo-controlled, challenge study in 62 healthy adult volunteers, the polymerase inhibitor ALS-008176, which selectively inhibits RSV RNA polymerase activity, resulted in a decreased viral load and an improvement of clinical disease severity in the treatment group [120]. However, a further phase 2 trial evaluating the antiviral activity and clinical outcomes in hospitalized infants and children showed an increase in reversible neutropenia and no antiviral activity [129].
6 Conclusion
RSV is the most common cause of LRTI in children, and has been associated with long-term pulmonary sequelae after infection, but despite this, treatment and preventative options have remained very limited. Recent successes, such as the licensure of nirsevimab as a preventative monoclonal antibody treatment for infants and the approval of two vaccines targeting RSV in the elderly and one in pregnant women, has led to renewed hope, with multiple other vaccine candidates also under examination. It remains only a question of time until the RSV landscape changes forever.
References
Levels & Trends in Child Mortality: Report 2020. Estimates developed by the United Nations Inter-agency Group for Child Mortality Estimation. New York: United Nations Children's Fund; 2020.
World Health Statistics 2019. Monitoring health for the SDGs, sustainable development goals. Geneva: World Health Organization; 2019.
Jain S, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–45.
Bhuiyan MU, et al. The contribution of viruses and bacteria to community-acquired pneumonia in vaccinated children: a case-control study. Thorax. 2019;74(3):261–9.
Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019;394(10200):757–79.
Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162(1–2):80–99.
Lee WJ, et al. Complete genome sequence of human respiratory syncytial virus genotype A with a 72-nucleotide duplication in the attachment protein G gene. J Virol. 2012;86(24):13810–1.
Bukreyev A, et al. The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. J Virol. 2008;82(24):12191–204.
Roberts SR, et al. The membrane-associated and secreted forms of the respiratory syncytial virus attachment glycoprotein G are synthesized from alternative initiation codons. J Virol. 1994;68(7):4538–46.
Hendricks DA, et al. Appearance of a soluble form of the G protein of respiratory syncytial virus in fluids of infected cells. J Gen Virol. 1987;68(Pt 6):1705–14.
McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol. 2013;372:83–104.
Swanson KA, et al. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci USA. 2011;108(23):9619–24.
Borchers AT, et al. Respiratory syncytial virus–a comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331–79.
Papenburg J, Boivin G. The distinguishing features of human metapneumovirus and respiratory syncytial virus. Rev Med Virol. 2010;20(4):245–60.
Glezen WP, et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–6.
Kutsaya A, et al. Prospective clinical and serological follow-up in early childhood reveals a high rate of subclinical RSV infection and a relatively high reinfection rate within the first 3 years of life. Epidemiol Infect. 2016;144(8):1622–33.
Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82(5):2040–55.
Boyce TG, et al. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137(6):865–70.
Hall CB, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132(2):e341–8.
Madhi SA, et al. Five-year cohort study of hospitalization for respiratory syncytial virus associated lower respiratory tract infection in African children. J Clin Virol. 2006;36(3):215–21.
Stensballe LG, et al. Atopic disposition, wheezing, and subsequent respiratory syncytial virus hospitalization in Danish children younger than 18 months: a nested case-control study. Pediatrics. 2006;118(5):e1360–8.
Fishaut M, Tubergen D, McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980;96(2):179–86.
Krinzman S, et al. Respiratory syncytial virus-associated infections in adult recipients of solid organ transplants. J Heart Lung Transplant. 1998;17(2):202–10.
Cohen C, et al. In- and out-of-hospital mortality associated with seasonal and pandemic influenza and respiratory syncytial virus in South Africa, 2009–2013. Clin Infect Dis. 2018;66(1):95–103.
Li Y, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–64.
Verwey, C., et al., Pulmonary function sequelae after respiratory syncytial virus lower respiratory tract infection in children: A systematic review. Pediatr Pulmonol, 2020.
Coutts J, et al. Association between respiratory syncytial virus hospitalization in infancy and childhood asthma. Pediatr Pulmonol. 2020;55(5):1104–10.
Escobar GJ, et al. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr. 2013;13:97.
Escobar GJ, et al. Recurrent wheezing in the third year of life among children born at 32 weeks’ gestation or later: relationship to laboratory-confirmed, medically attended infection with respiratory syncytial virus during the first year of life. Arch Pediatr Adolesc Med. 2010;164(10):915–22.
Schauer U, et al. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur Respir J. 2002;20(5):1277–83.
Pullan CR, Hey EN. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br Med J (Clin Res Ed). 1982;284(6330):1665–9.
Stein RT, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354(9178):541–5.
Sigurs N, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045–52.
Sigurs N, et al. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161(5):1501–7.
Sigurs N, et al. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95(4):500–5.
Sigurs N, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171(2):137–41.
Hall CB. Respiratory syncytial virus: its transmission in the hospital environment. Yale J Biol Med. 1982;55(3–4):219–23.
Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev. 2014;35(12):519–30.
Johnson KM, et al. Respiratory syncytial virus. IV. Correlation of virus shedding, serologic response, and illness in adult volunteers. JAMA. 1961;176:663–7.
Shigeta S, et al. The cell to cell infection of respiratory syncytial virus in HEp-2 monolayer cultures. J Gen Virol. 1968;3(1):129–31.
Aherne W, et al. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970;23(1):7–18.
Christiaansen AF, et al. The CD4 T cell response to respiratory syncytial virus infection. Immunol Res. 2014;59(1–3):109–17.
Openshaw PJM, et al. Protective and harmful immunity to RSV infection. Annu Rev Immunol. 2017;35:501–32.
Chiu C, Openshaw PJ. Antiviral B cell and T cell immunity in the lungs. Nat Immunol. 2015;16(1):18–26.
Habibi MS, et al. Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am J Respir Crit Care Med. 2015;191(9):1040–9.
Ogilvie MM, et al. Maternal antibody and respiratory syncytial virus infection in infancy. J Med Virol. 1981;7(4):263–71.
Ochola R, et al. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS ONE. 2009;4(12): e8088.
Buchwald AG, et al. Respiratory syncytial virus (RSV) neutralizing antibodies at birth predict protection from RSV illness in infants in the first 3 months of life. Clin Infect Dis. 2021;73(11):e4421–7.
de Sierra TM, et al. Respiratory syncytial virus-specific immunoglobulins in preterm infants. J Pediatr. 1993;122(5 Pt 1):787–91.
Sande CJ, Cane PA, Nokes DJ. The association between age and the development of respiratory syncytial virus neutralising antibody responses following natural infection in infants. Vaccine. 2014;32(37):4726–9.
Spann KM, Tran KC, Collins PL. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J Virol. 2005;79(9):5353–62.
Rallabhandi P, et al. Respiratory syncytial virus fusion protein-induced Toll-like receptor 4 (TLR4) signaling is inhibited by the TLR4 antagonists Rhodobacter sphaeroides lipopolysaccharide and eritoran (E5564) and requires direct interaction with MD-2. MBio. 2012;3(4):10–1128.
Jeong KI, et al. CX3CR1 is expressed in differentiated human ciliated airway cells and co-localizes with respiratory syncytial virus on cilia in a G protein-dependent manner. PLoS ONE. 2015;10(6): e0130517.
McLellan JS. Neutralizing epitopes on the respiratory syncytial virus fusion glycoprotein. Curr Opin Virol. 2015;11:70–5.
Siber GR, et al. Protective activity of a human respiratory syncytial virus immune globulin prepared from donors screened by microneutralization assay. J Infect Dis. 1992;165(3):456–63.
Groothuis JR, Simoes EA, Hemming VG. Respiratory syncytial virus (RSV) infection in preterm infants and the protective effects of RSV immune globulin (RSVIG). Respiratory Syncytial Virus Immune Globulin Study Group. Pediatrics. 1995;95(4):463–7.
Groothuis JR, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993;329(21):1524–30.
Simoes EA, et al. Respiratory syncytial virus-enriched globulin for the prevention of acute otitis media in high risk children. J Pediatr. 1996;129(2):214–9.
Johnson S, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176(5):1215–24.
Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102(3 Pt 1):531–7.
Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2): 415–20.
Shahabi A, et al. Assessing variation in the cost of palivizumab for respiratory syncytial virus prevention in preterm infants. Pharmacoecon Open. 2018;2(1):53–61.
Caserta MT, et al. Palivizumab prophylaxis in infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2023.
Caserta MT, et al. Palivizumab prophylaxis in infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2023;152(1).
Reeves RM, et al. A systematic review of European clinical practice guidelines for respiratory syncytial virus prophylaxis. J Infect Dis. 2022;226(Suppl 1):S110-s116.
Stiboy E, Chan M. Variation in clinical practice guidelines for use of palivizumab in preventing severe respiratory syncytial viral (RSV) disease in high-risk infants. Pediatr Pulmonol. 2023;58(4):1210–20.
Obando-Pacheco P, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217(9):1356–64.
Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem. 2006;281(33):23514–24.
Wu H, et al. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368(3):652–65.
Carbonell-Estrany X, et al. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125(1):e35-51.
FDA Panel Nixes Licensing Request for Motavizumab. 2010 [cited 2022 October, 2022]. https://www.medscape.com/viewarticle/722903.
Simões EAF, et al. Suptavumab for the prevention of medically attended respiratory syncytial virus infection in preterm infants. Clin Infect Dis. 2021;73(11):e4400–8.
Zhu Q, McLellan JS. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med. 2017;9(388): eaaj1928.
Griffin MP, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383(5):415–25.
Hammitt LL, Dagan R. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386(9):837–46.
Dagan R, H L, Nunez BS, Cots MB, Bosheva M, Madhi SA, Muller WJ, Zar HJ, Grenham A, Shroff M, Takas T, Mankad VS, Leach A, Villafana T. Nirsevimab for the prevention of RSV disease in healthy late-preterm and term infants: follow-up through second RSV season. In: 12th international RSV symposium. 2022.
Simões EAF, et al. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: a pooled analysis of randomised controlled trials. Lancet Child Adolesc Health. 2023;7(3):180–9.
Kim HW, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–34.
Bigay J, et al. Vaccine-associated enhanced disease in humans and animal models: Lessons and challenges for vaccine development. Front Microbiol. 2022;13: 932408.
Polack FP, et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med. 2002;196(6):859–65.
PATH. RSV Vaccine and mAb Snapshot. 2021 September 2021 [cited 2022 02 September 2022]. https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/.
Graham BS. Vaccines against respiratory syncytial virus: the time has finally come. Vaccine. 2016;34(30):3535–41.
Madhi SA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383(5):426–39.
Furuta M, et al. Efficacy and safety of pertussis vaccination for pregnant women—a systematic review of randomised controlled trials and observational studies. BMC Pregnancy Childbirth. 2017;17(1):390.
Benowitz I, et al. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis. 2010;51(12):1355–61.
Nunes MC, et al. Effectiveness of influenza vaccination of pregnant women for prevention of maternal and early infant influenza-associated hospitalizations in South Africa: a prospective test-negative study. 2022;9(11):552.
Nunes MC, et al. Efficacy of maternal influenza vaccination against all-cause lower respiratory tract infection hospitalizations in young infants: results from a randomized controlled trial. Clin Infect Dis. 2017;65(7):1066–71.
Wright PF, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182(5):1331–42.
Karron RA, et al. Live-attenuated vaccines prevent respiratory syncytial virus-associated illness in young children. Am J Respir Crit Care Med. 2021;203(5):594–603.
ClinicalTrials.gov. 2022 [cited 2022 05 September 2022]. https://www.clinicaltrials.gov/ct2/home.
Walsh EE, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465–77.
Papi A, Ison MG. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med. 2023;388(7):595–608.
GSK provides further update on phase III RSV maternal vaccine candidate programme. 2022 [cited 2022 16 November 2022]. https://www.gsk.com/en-gb/media/press-releases/gsk-provides-further-update-on-phase-iii-rsv-maternal-vaccine-candidate-programme/.
Bebia Z, et al. Safety and immunogenicity of an investigational respiratory syncytial virus vaccine (RSVPreF3) in mothers and their infants: a phase 2 randomized trial. 2023.
Pfizer Announces Positive Top-Line Data of Phase 3 Global Maternal Immunization Trial for its Bivalent Respiratory Syncytial Virus (RSV) Vaccine Candidate. 2022 [cited 2022 04 November]. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-phase-3-global.
Kampmann B, Madhi SA. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388(16):1451–64.
Pfizer I. Vaccines and related biological products advisory Committee Meeting, May 18, 2023: Respiratory Syncytial Virus Vaccine (Proposed Trade Name: Abrysvo). 2023.
Dolgin E. The tangled history of mRNA vaccines. Nature. 2021;597(7876):318–24.
Pardi N, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–79.
Bisgaard H, et al. Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children. Am J Respir Crit Care Med. 2008;178(8):854–60.
Bulow SM, et al. Prednisolone treatment of respiratory syncytial virus infection: a randomized controlled trial of 147 infants. Pediatrics. 1999;104(6): e77.
Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–93.
Steiner RW. Treating acute bronchiolitis associated with RSV. Am Fam Physician. 2004;69(2):325–30.
Buckingham SC, et al. A randomized, double-blind, placebo-controlled trial of dexamethasone in severe respiratory syncytial virus (RSV) infection: effects on RSV quantity and clinical outcome. J Infect Dis. 2002;185(9):1222–8.
Cade A, et al. Randomised placebo controlled trial of nebulised corticosteroids in acute respiratory syncytial viral bronchiolitis. Arch Dis Child. 2000;82(2):126–30.
Hammer J, Numa A, Newth CJ. Albuterol responsiveness in infants with respiratory failure caused by respiratory syncytial virus infection. J Pediatr. 1995;127(3):485–90.
Proesmans M, et al. Montelukast does not prevent reactive airway disease in young children hospitalized for RSV bronchiolitis. Acta Paediatr. 2009;98(11):1830–4.
Rodriguez WJ, et al. Respiratory syncytial virus immune globulin treatment of RSV lower respiratory tract infection in previously healthy children. Pediatrics. 1997;100(6):937–42.
Roosevelt G, et al. Dexamethasone in bronchiolitis: a randomised controlled trial. Lancet. 1996;348(9023):292–5.
van Woensel JB, et al. Randomised double blind placebo controlled trial of prednisolone in children admitted to hospital with respiratory syncytial virus bronchiolitis. Thorax. 1997;52(7):634–7.
Dalziel SR, et al. Bronchiolitis. Lancet. 2022;400(10349):392–406.
DeVincenzo JP. Therapy of respiratory syncytial virus infection. Pediatr Infect Dis J. 2000;19(8):786–90 (discussion 802–804, 811–813).
Thomas E, Ghany MG, Liang TJ. The application and mechanism of action of ribavirin in therapy of hepatitis C. Antivir Chem Chemother. 2012;23(1):1–12.
Tejada S, et al. Ribavirin for treatment of subjects with respiratory syncytial virus-related infection: a systematic review and meta-analysis. Adv Ther. 2022;39(9):4037–51.
Foolad F, et al. Oral versus aerosolized ribavirin for the treatment of respiratory syncytial virus infections in hematopoietic cell transplant recipients. Clin Infect Dis. 2019;68(10):1641–9.
Permpalung N, et al. Oral and inhaled ribavirin treatment for respiratory syncytial virus infection in lung transplant recipients. Transplantation. 2020;104(6):1280–6.
Beaird OE, Freifeld A, Ison MG. Current practices for treatment of respiratory syncytial virus and other non-influenza respiratory viruses in high-risk patient populations: a survey of institutions in the Midwestern Respiratory Virus Collaborative. Transpl Infect Dis. 2016;18(2):210–5.
DeVincenzo J, et al. Safety and antiviral effects of nebulized PC786 in a respiratory syncytial virus challenge study. J Infect Dis. 2022;225(12):2087–96.
DeVincenzo JP, et al. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med. 2014;371(8):711–22.
DeVincenzo JP, et al. Activity of oral ALS-008176 in a respiratory syncytial virus challenge study. N Engl J Med. 2015;373(21):2048–58.
Stevens M, et al. Antiviral activity of oral JNJ-53718678 in healthy adult volunteers challenged with respiratory syncytial virus: a placebo-controlled study. J Infect Dis. 2018;218(5):748–56.
DeVincenzo J, et al. A randomized, placebo-controlled, respiratory syncytial virus human challenge study of the antiviral efficacy, safety, and pharmacokinetics of RV521, an inhibitor of the RSV-F protein. Antimicrob Agents Chemother. 2020;64(2):10–128.
Detalle L, et al. Generation and characterization of ALX-0171, a potent novel therapeutic nanobody for the treatment of respiratory syncytial virus infection. Antimicrob Agents Chemother. 2016;60(1):6–13.
Van Heeke G, et al. Nanobodies® as inhaled biotherapeutics for lung diseases. Pharmacol Ther. 2017;169:47–56.
Cunningham S, et al. Nebulised ALX-0171 for respiratory syncytial virus lower respiratory tract infection in hospitalised children: a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med. 2021;9(1):21–32.
Battles MB, et al. Molecular mechanism of respiratory syncytial virus fusion inhibitors. Nat Chem Biol. 2016;12(2):87–93.
Marty FM, et al. A phase 2b, randomized, double-blind, placebo-controlled multicenter study evaluating antiviral effects, pharmacokinetics, safety, and tolerability of presatovir in hematopoietic cell transplant recipients with respiratory syncytial virus infection of the lower respiratory tract. Clin Infect Dis. 2020;71(11):2787–95.
Martinón-Torres F, et al. Pharmacokinetics, safety, and antiviral effects of multiple doses of the respiratory syncytial virus (RSV) fusion protein inhibitor, JNJ-53718678, in infants hospitalized with RSV infection: a randomized phase 1b study. Clin Infect Dis. 2020;71(10):e594–603.
Oey A, et al. Lumicitabine, an orally administered nucleoside analog, in infants hospitalized with respiratory syncytial virus (RSV) infection: safety, efficacy, and pharmacokinetic results. PLoS ONE. 2023;18(7): e0288271.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by University of the Witwatersrand. CV: Previously received personal funding from AstraZeneca, Merck, Cipla, and GSK. SAM and ZD: Grant support to institution from BMGF; involved in clinical trials with Pfizer, GSK, Merck, and AstraZeneca, with funding to institution. CV, ZD, and SAM are involved with a clinical trial for Enanta Pharmaceuticals. No funding was received for the preparation of this article.
Conflict of interest
CV, SAM, and ZD declare no conflicts of interest related to this article.
Data availability
All data represented are available in the public domain.
Ethics approval
No ethics approval was required.
Consent to participate
Patient consent not applicable.
Author contributions
This article is the sole work of the authors. CV, SAM, and ZD contributed equally to the planning, development, writing, and final approval of the manuscript.
Consent for publication
Not applicable
Code availability
Not applicable
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Verwey, C., Dangor, Z. & Madhi, S.A. Approaches to the Prevention and Treatment of Respiratory Syncytial Virus Infection in Children: Rationale and Progress to Date. Pediatr Drugs 26, 101–112 (2024). https://doi.org/10.1007/s40272-023-00606-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00606-6