Abstract
Locus coeruleus (LC) is the main noradrenergic nucleus of the brain, and degenerates early in Parkinson’s disease (PD). The objective of this study is to test whether degeneration of the LC is associated with orthostatic hypotension (OH) in PD. A total of 22 cognitively intact PD patients and 52 age-matched healthy volunteers underwent 3 T magnetic resonance (MRI) with neuromelanin-sensitive T1-weighted sequences (LC-MRI). For each subject, a template space-based LC-MRI was used to calculate LC signal intensity (LC contrast ratio—LCCR) and the estimated number of voxels (LCVOX) belonging to LC. Then, we compared the LC-MRI parameters in PD patients with OH (PDOH+) versus without OH (PDOH−) (matched for sex, age, and disease duration) using one-way analysis of variance followed by multiple comparison tests. We also tested for correlations between subject’s LC-MRI features and orthostatic drop in systolic blood pressure (SBP). PDOH− and PDOH+ did not differ significantly (p > 0.05) based on demographics and clinical characteristics, except for blood pressure measurements and SCOPA-AUT cardiovascular domain (p < 0.05). LCCR and LCVOX measures were significantly lower in PD compared to HC, while no differences were observed between PDOH− and PDOH+. Additionally, no correlation was found between the LC-MRI parameters and the orthostatic drop in SBP or the clinical severity of autonomic symptoms (p > 0.05). Conversely, RBD symptom severity negatively correlated with several LC-MRI parameters. Our results failed to indicate a link between the LC-MRI features and the presence of OH in PD but confirmed a marked alteration of LC signal in PD patients.
Similar content being viewed by others
Introduction
The locus coeruleus (LC) is the main noradrenergic (NA) nucleus of the brain, giving rise to diffuse projections throughout the whole central nervous system (CNS) (Poe et al. 2020).
Extensive neuropathological evidence in humans indicates that there is a marked LC neuronal loss in Parkinson’s disease (PD) and that a significant LC degeneration occurs since the early stages of disease, years before the onset of motor symptoms (Iranzo et al. 2014; Oertel et al. 2019).
However, LC degeneration estimation in vivo has been obtained only recently by magnetic resonance imaging (MRI) with LC-sensitive sequences (LC-MRI) (Sasaki et al. 2006).
LC dysfunction is associated with several non-motor symptoms of PD, such as anxiety, depression, REM behavior disorder (RBD), cognitive disturbances, apathy, and fatigue (Remy et al. 2005; Tredici and Braak 2013).
Orthostatic hypotension (OH) is a common and debilitating non-motor manifestation of PD with a point prevalence of 30% (Velseboer et al. 2011). The presence of OH in early disease stages is associated with a poor prognosis, clustering with cognitive deficits and RBD in a malignant phenotype of PD (Fereshtehnejad et al. 2015). Importantly, RBD and impaired cognitive functioning have been both related to LC dysfunction as underlying mechanism (Paredes-Rodriguez et al. 2020). Thus, it is not surprising that, although the causes of OH in PD are multifactorial, there is increasing evidence that NA dysfunction plays a prominent role. In particular, although OH is classically thought to be mainly the result of a NA cardiac denervation and peripheral NA deficiency, accompanied by impaired baroreflexes (Jain and Goldstein 2012), a damage to the LC complex has been also strongly related to its occurrence (Sommerauer et al. 2018).
MRI has been successfully used to study LC integrity, but only very recently a more standardized methodological framework for LC imaging analysis has been suggested (Betts et al. 2019; Giorgi et al. 2022; Galgani et al. 2023).
In this study, we hypothesize that the central NA system is more severely affected in PD patients with OH (PDOH+) compared to PD patients without OH (PDOH−), and thus, we analyzed LC through MRI in these two groups of subjects.
Materials and methods
Twenty-two patients with PD were specifically enrolled for this study at the Center for Parkinson’s Disease and Movement Disorders of the Unit of Neurology of the University of Pisa, while 53 age- and sex-matched cognitively intact and neurologically healthy controls (HC) were part of a previously published study (Galgani et al. 2023). The LC-MRI protocol was approved by the Ethical Committee of Pisa University Hospital, which was conducted in accordance with Helsinki Declaration, and written informed consent was obtained before LC-MRI (prot.#1203, PE-2013–02349574). The diagnosis of PD was made according to the MDS clinical diagnostic criteria for Parkinson’s disease (Postuma et al. 2015). Other inclusion criteria were as follows: Hoehn and Yahr scales between 1 and 3 and stable dosage of dopaminergic medications for at least 4 weeks before the evaluation. We excluded patients with dementia, according to current consensus clinical diagnostic criteria (Emre et al. 2007).
Firstly, we included patients with OH (PDOH+), defined as a fall in systolic blood pressure (SBP) ≥ 20 mmHg or diastolic BP (DBP) ≥ 10 mmHg within 3 min of standing. Measurement of supine and standing heart rate (HR) was used to confirm that patients had neurogenic OH (nOH) (ΔHR/ΔSBP ratio lower than 0.5 bpm/mmHg after 3 min in the standing position) (Norcliffe-Kaufmann et al. 2018; Guaraldi et al. 2020). Baseline blood pressure was defined as the mean of two measurements on the upper right arm with the participant in the supine position after 5 min of rest. The measurements were repeated in a standing position after 1, 3, and 5 min. We adopted stringent exclusion criteria to account for as many potentially existing confounding variables as possible for OH, excluding eventually candidates (among both patients and HC) with diabetes, hypertension, and other disorders potentially associated with autonomic dysfunction, severe intracranial or extracranial artery stenosis/occlusion, clinical history of acute cerebrovascular disease, history of peripheral arterial disease, or taking medications for heart or other drugs such as tricyclic antidepressants and alpha-adrenergic antagonists (e.g., for prostate disorders) that influence orthostatic challenge. Other general exclusion criteria were severe medical comorbidities, psychiatric illnesses, and MRI signs of moderate–severe chronic vascular encephalopathy, according to Fazekas et al. (1987).
Then, for every PD patient with nOH, we looked for PD subjects without OH (PDOH) but with similar age (± 2 years) and disease duration (± 2 years) and matched by gender with the abovementioned PDOH+ subjects.
Each patient underwent full neurological examination at the time of BP assessment. Motor symptoms were assessed with the Unified Parkinson’s Disease Rating Scale (UPDRS III). The MOntreal Cognitive Assessment (MoCA), SCales for Outcomes in Parkinson’s Disease (SCOPA), Hamilton Anxiety Rating Scale (HAM-A), Hamilton Depression Rating Scale (HDRS), and REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ) were also collected.
MRI protocol
LC imaging was performed using a 3-Tesla MRI unit (GE Excite HDx, GE, USA) with an 8-channel phased-array head coil. A 2D-FSE T1-weighted sequence was registered with the following parameters: TR, 600 ms; TE, 14 ms; flip angle, 90°; echo train length, 2; NEX, 5; matrix size, 512 × 384; FOV, 200 × 200 mm; pixel size, 0.39 × 0.52 mm; contiguous slices, 12, slice thickness, 2.2 mm, slice gap, 0; and acquisition time, 14.29 min). The images were acquired at the level of the anatomical location of LC (Fernandes et al. 2012), covering an area from the inferior border of the pons to the posterior commissure, along the oblique axial plane and perpendicular to the fourth ventricle floor.
The post-acquisition analysis was performed profiting from a study-specific template, whose detailed description is reported in (Giorgi et al. 2022) and (Galgani et al. 2023). The scans of patients were warped from the native space to the template space, and LC mask was applied. We calculated the LC contrast ratio “LCCR” (i.e., the ratio between the intensity detected within the LC mask and the one of the reference pontine regions), as an index of LC signal intensity, and “LCVOX” as an estimate of LC voxels belonging to LC. For each subject, we calculated the LCCR and LCVOX of the entire LC both for left (“left LC”) and right (“right LC”) hemispheres.
Statistical analysis
Demographics and clinical variables were compared between PDOH+ and PDOH− using an independent t-test (p < 0.05). The data were first tested for normality with the Kolmogorov–Smirnov test. Because most MRI data exhibited normal distributions, parametric statistics were applied. Between-group differences in LCCR and LCVOX measures were tested using parametric one-way ANOVA followed by post hoc analysis. Partial Pearson correlation coefficient analysis was used to assess associations between LCCR/LCVOX parameters and blood pressure values as well as between LCCR/LCVOX and the other motor and non-motor symptoms (depression, anxiety, RBD, cognition, and other dysautonomic symptoms than OH) evaluated at the time of BP assessment. Statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS), version 26.
Results
Demographics and clinical assessment
Demographics and baseline clinical characteristics of the population included in this study are reported in Table 1. Twenty-two PD subjects were recruited. However, one PDOH− patient was excluded from the final analysis due to MRI movement artifacts. PD groups were matched for age, sex, and disease duration. Among all PD participants, the mean (SD) age was 71.4 (5.4) years and mean (SD) disease duration was 6.2 (3.4) years. There were no differences in LEDD, and disease severity between PDOH+ and PDOH− and both groups had similar scores in non-motor scales (MoCA, SCOPA-AUT total score, HAM-A, HDRS, and RBDSQ). As expected, PDOH+ had significant SBP and DBP than PDOH+ and worse scores in the cardiovascular domain of the SCOPA-AUT scale (Table 1).
LC group differences
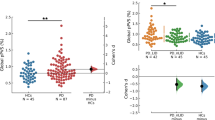
ANOVA analysis revealed a statistically significant difference in LCCR and LCVOX among the three groups both for the right LC and the left LC (Fig. 1). In particular, both PD groups had significantly lower LCCR and LCVOX than HC group (a representative image of two subjects is reported in Fig. 2), but there was no significant difference in LC parameters between PDOH+ and PDOH− (Fig. 1). We obtained the same results when performed comparison on LCCR and LCVOX for each (rostral and caudal) subregion of the LC (data not shown).
Representative locus coeruleus (LC)–magnetic resonance imaging (LC-MRI) images from PD and healthy controls (HC). The figure shows representative images from 2D-FSE T1-weighted sequence in native space (before post-acquisition LC data extraction, see methods) in corresponding slices at the level of the pons, from one PD and one HC. In each slice, a box insert shows the region where the two LC are placed (visualized just laterally to the median plane, below the floor of the fourth ventricle). In PD, there is a marked decrease in LC-related intensity compared with HC
Relation between LC parameters and clinical variables
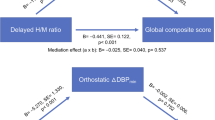
Linear correlation analysis in the included PD subjects did not show any relevant correlation between the single subject’s LC-MRI parameters and neither the drop in blood pressure nor the SCOPA-AUT total score and domain scores. Conversely, RBD symptom severity negatively correlated with LC-MRI parameters (Fig. 3).
Discussion
In this study, we explored the under-investigated link between degeneration of LC and OH in PD through MRI, and our data suggest that LC degeneration is not strictly associated with nOH. Our findings indicate that LC is significantly altered in all PD patients when compared with healthy individuals, but no differences in the PD population were found with regard to the presence of OH. Conversely, in line with our predictions, we found evidence for a link between RBD score and LC degeneration.
The exact pathophysiology of OH in PD is incompletely understood. Patients with PD tend to demonstrate predominantly peripheral autonomic failure (Coon 2020). Defective NA release from postganglionic sympathetic neurons and impaired compensatory baroreflex response are thought to be the primary pathophysiological mechanisms of nOH in PD (Jain and Goldstein 2012). Moreover, deposition of alpha-synuclein (α-syn) along the central autonomic network may also contribute to nOH (Coon et al. 2018). The nucleus of the solitary tract and rostral ventrolateral medulla have been shown to play an important role in the activation of the baroreflex system, receiving the modulation, among others, of the LC itself through both direct and indirect connections (Bockstaele et al. 1989; Sun 1995; Giorgi et al. 2021; Samuels and Szabadi 2008). Although the LC is among the first brain regions to degenerate in PD (Braak et al. 2003), few studies examined the structural degeneration of LC in the pathogenesis of PD non-motor symptoms (Malatt and Tagliati 2022), likely reflecting previous technical difficulties in visualizing in vivo the LC because of its small size and physiological inter-subject variability (Fernandes et al. 2012). However, in the last decade, LC-MRI data have been shown to be able to provide a quite reliable surrogate for LC integrity determined histopathologically (Keren et al. 2015), after the improvement of specific MRI sequences, and the development of standardized analysis protocols. Specifically, the combination of neuromelanin with ions and macromolecules within LC neurons likely contributes to a T1-shortening effect in fast spin-echo sequences and to T1 prolongation at magnetization transfer-weighted imaging (Galgani et al. 2020). In PD, a significant reduction of LC-MRI intensity has been confirmed (Castellanos et al. 2015; Schwarz et al. 2017), even greater than that the substantia nigra MRI one (Isaias et al. 2016).
Our results confirmed both a significant reduction of LC-MRI signal intensity in PD patients versus controls and the lack of correlation between MRI-based LC parameters and disease duration or motor severity (Ohtsuka et al. 2014; Prasuhn et al. 2021; Doppler et al. 2021). Nevertheless, LC analysis may be theoretically useful for identifying patients suffering from non-motor symptoms. It shows an association between altered LC signal and RBD (García-Lorenzo et al. 2013; Knudsen et al. 2018), depression (Solopchuk et al. 2018; Wang et al. 2018, Ye et al. 2022; Madelung et al. 2022), and cognitive dysfunction (Prasuhn et al. 2021; Li et al. 2019). Interestingly, regionally specific pattern of LC changes in PD, with the middle-caudal portion being more affected than the rostral part, has been associated with cognitive impairment and apathy (Ye et al. 2022; Madelung et al. 2022). A regional association with the left caudal LC has been also found for OH in 42 PD patients (of whom 17 had OH) in a recent study investigating the potential link between orthostatic BP drop and LC-MRI signal changes (Madelung et al. 2022). We failed to find an association between OH and LC-MRI parameters in the present study, which was specifically designed to test the hypothesis of a different LC-MRI signal in PD patients with and without nOH, matched for age, sex and disease duration. The same held true when we tested for regional (rostral and caudal) differences in neurodegeneration within the LC, in line with postmortem studies reporting a uniform neuronal loss over the entire LC in PD (German et al. 1992). Besides, we did not see an overall significant association between LC integrity and the severity of other non-motor symptoms (depression, anxiety, and cognition), except for RBD. This finding is not unexpected since LC-MRI signal intensity has been shown to be significantly decreased even in RBD patients without PD (Ehrminger et al. 2016). Interestingly, in the present study, the relationship between RBD and LC was confirmed both for the caudal and rostral portion of LC (not shown).
RBD often co-occurs with poor cognition and OH, together contributing to define a rapidly progressive subtype of PD whose biological underpinning could result from NA deficiency (Espay et al. 2014). Sommerauer and colleagues (2018) tested this hypothesis in a combined 11C-MeNER (a radioligand specific for NA transporter) PET and LC-MRI study in PD patients with and without RBD and healthy controls. They detected that there is an association between the NA system as measured with 11C-MeNER PET and all three groups and that PD patients with RBD displayed the lowest neuromelanin signal in LC; however, LC-MRI signal did not significantly correlate neither with RBD, nor with cognitive performance or with BP changes (Sommerauer et al. 2018). Similarly, we did not find a relationship between LC degeneration and orthostatic drop in BP, and LC-MRI signal was significantly lower in all PD patients versus controls, without differences between patients with and without OH, in line with a recent neuropathological study in forty-four patients with PD/Lewy body disease which failed to indicate an association of LC pathology with the presence of OH (Tong and Chen 2021). One potential explanation for these negative imaging and pathological findings might be the inclusion in the above-reported studies of subjects with intermediate/advanced disease stage, as LC degeneration in PD starts early, and thus, LC-MRI may potentially disclose differences among those PD subgroups at the early stage only or even in the prodromal phases of the disease. In fact, LC could already be significantly damaged when motor symptoms firstly appear (Braak et al. 2003) This may account for the failure of LC-MRI to distinguish between early and advanced PD (Ohtsuka et al. 2014) and to identify, as in our study, differences between PD subgroups in a cohort evaluated after a mean disease duration of follow-up of 6 years.
In contrast, NA terminal integrity, measured through 11C-MeNER PET, has been linked to the expression of OH and other clinical PD symptoms more consistently than LC nuclear integrity assessed with LC-MRI (Sommerauer et al. 2018; Kelberman et al. 2020). A disease process beginning in the distal axon and proceeding retrogradely (similarly to what was proposed for the dopaminergic nigrostriatal pathway degeneration (Cheng et al. 2010)) could explain, at least in part, the discrepancy between structural and functional imaging modalities in studying the NA neurons of the LC itself. However, adaptive circuit changes, the heterogeneity among LC projection neurons, and their large axonal arborization are other factors potentially contributing to this discrepancy. In addition, it appears that LC-MRI and PET findings are not closely correlated even in healthy subjects, suggesting that the two compartments of the NA system (cell bodies and axon terminals) could be independent of each other (Helmich and Lehéricy 2021).
In contrast to our study, none of the previous ones distinguished between nOH and non-nOH. Moreover, we included only PDOH+ who had never taken any drugs affecting autonomic function and without comorbidities potentially contributing to autonomic disturbances. However, several limitations, such as the small sample size of PD patients and the single-center nature of the study, should be acknowledged. The relatively small number of participants with PD did not allow performing additional analyses, but these numbers are aligned with those of the only two studies published thus far on the relationship between LC-MRI signal intensity and OH (Sommerauer et al. 2018; Madelung et al. 2022). Besides, we acknowledge that the cardiovascular autonomic assessment was not comprehensive since it was limited to the study of BP and heart rate, while RBD was measured only through the RBD questionnaire and not by polysomnography. Furthermore, although there was no significant difference in LEDD between PDOH+ and PDOH−, the impact of anti-PD medication on OH cannot be ruled out. Finally, it is worth mentioning that in our study the HC group had been included exclusively for comparison of LC-MRI with PD groups, but, since BP parameters had not been systematically assessed in these HC subjects, we could not perform an association analysis of LC-MRI and BP parameters along the whole spectrum of LC degeneration; however, this did not affect the main purpose of the analysis which was based on the comparison between PDOH+ and PDOH−.
In conclusion, we did not find any difference in LC-MRI parameters in PD patients with and without neurogenic OH, while we confirmed a significant LC degeneration in all PD patients compared with controls, suggesting that changes in LC signal might not be useful for identifying PD patients with cardiovascular dysautonomia, at least at this stage of the disease. Future studies with larger and early/prodromal PD cohorts with longitudinal follow-up will provide greater insights about the potential link between the integrity of LC and OH.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Betts MJ, Kirilina E, Otaduy MCG, Ivanov D, Acosta-Cabronero J, Callaghan MF et al (2019) Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain 142(9):2558–2571
Braak H, del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Castellanos G, Fernández-Seara MA, Lorenzo-Betancor O, Ortega-Cubero S, Puigvert M, Uranga J et al (2015) Automated neuromelanin imaging as a diagnostic biomarker for Parkinson’s disease. Mov Disord 30(7):945–952
Cheng HC, Ulane CM, Burke RE (2010) Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 67(6):715–725
Coon EA (2020) Autonomic Dysfunction in the Synucleinopathies. Semin Neurol 40(5):492–501
Coon EA, Cutsforth-Gregory JK, Benarroch EE (2018) Neuropathology of autonomic dysfunction in synucleinopathies. Mov Disord 33(3):349–358
Del Tredici K, Braak H (2013) Dysfunction of the locus coeruleus norepinephrine system and related circuitry in Parkinson’s disease-related dementia. J Neurol Neurosurg Psychiatry 84(7):774–783
Doppler CEJ, Kinnerup MB, Brune C, Farrher E, Betts M, Fedorova TD et al (2021) Regional locus coeruleus degeneration is uncoupled from noradrenergic terminal loss in Parkinson’s disease. Brain 144(9):2732–2744
Ehrminger M, Latimier A, Pyatigorskaya N, Garcia-Lorenzo D, Leu-Semenescu S, Vidailhet M et al (2016) The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behavior disorder. Brain 139(4):1180–1188
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y et al (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22(12):1689–1837
Espay AJ, LeWitt PA, Kaufmann H (2014) Norepinephrine deficiency in Parkinson’s disease: the case for noradrenergic enhancement. Mov Disord 29(14):1710–1719
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149(2):351–356
Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB (2015) New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression: A Prospective Cohort Comparison With Other Phenotypes. JAMA Neurol 72(8):863–873
Fernandes P, Regala J, Correia F, Gonçalves-Ferreira AJ (2012) The human locus coeruleus 3-D stereotactic anatomy. Surg Radiol Anat 34(10):879–885
Galgani A, Lombardo F, Della Latta D, Martini N, Bonuccelli U, Fornai F, Giorgi FS (2020) Locus Coeruleus Magnetic Resonance Imaging in Neurological Diseases. Curr Neurol Neurosci Rep 21(1):2
Galgani A, Lombardo F, Martini N, Vergallo A, Bastiani L, Hampel H et al (2023) Magnetic resonance imaging Locus Coeruleus abnormality in amnestic Mild Cognitive Impairment is associated with future progression to dementia. Eur J Neurol 30(1):32–46
García-Lorenzo D, Longo-Dos Santos C, Ewenczyk C, Leu-Semenescu S, Gallea C et al (2013) The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson’s disease. Brain 136(7):2120–2129
German DC, Manaye KF, White CL 3rd, Woodward DJ, McIntire DD, Smith WK et al (1992) Disease-specific patterns of locus coeruleus cell loss. Ann Neurol 32:667–676
Giorgi FS, Galgani A, Puglisi-Allegra S, Busceti CL, Fornai F (2021) The connections of Locus Coeruleus with hypothalamus: potential involvement in Alzheimer’s disease. J Neural Transm (vienna) 128(5):589–613
Giorgi FS, Martini N, Lombardo F, Galgani A, Bastiani L, Della Latta D et al (2022) Locus Coeruleus magnetic resonance imaging: a comparison between native-space and template-space approach. J Neural Transm (vienna) 29(4):387–394
Guaraldi P, Baschieri F, Barletta G, Cecere A, Cortelli P, Calandra-Buonaura G (2020) Validation of the new index of baroreflex function to identify neurogenic orthostatic hypotension. Auton Neurosci 229:102744
Helmich RC, Lehéricy S (2021) Dying-back of ascending noradrenergic projections in Parkinson’s disease. Brain 144(9):2562–2564
Iranzo A, Gelpi E, Tolosa E, Molinuevo JL, Serradell M, Gaig C, Santamaria J (2014) Neuropathology of prodromal Lewy body disease. Mov Disord 29(3):410–415
Isaias IU, Trujillo P, Summers P, Marotta G, Mainardi L, Pezzoli G et al (2016) Neuromelanin imaging and dopaminergic loss in Parkinson’s disease. Front Aging Neurosci 8:196
Jain S, Goldstein DS (2012) Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol Dis 46(3):572–580
Kelberman M, Keilholz S, Weinshenker D (2020) What’s That (Blue) Spot on my MRI? Multimodal Neuroimaging of the Locus Coeruleus in Neurodegenerative Disease. Front Neurosci 14:583421
Keren NI, Taheri S, Vazey EM, Morgan PS, Granholm ACE, Aston-Jones GS et al (2015) Histologic validation of locus coeruleus MRI contrast in post-mortem tissue. Neuroimage 113:235–245
Knudsen K, Fedorova TD, Hansen AK, Sommerauer M, Otto M, Svendsen KB et al (2018) In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol 17(7):618–628
Li Y, Wang C, Wang J, Zhou Y, Ye F, Zhang Y et al (2019) Mild cognitive impairment in de novo Parkinson’s disease: A neuromelanin MRI study in locus coeruleus. Mov Disord 34(6):884–892
Madelung CF, Meder D, Fuglsang SA, Marques MM, Boer VO, Madsen KH et al (2022) Locus Coeruleus Shows a Spatial Pattern of Structural Disintegration in Parkinson’s Disease. Mov Disord 37(3):479–489
Malatt C, Tagliati M (2022) The role of the locus coeruleus/norepinephrine system in the pathogenesis of neurodegenerative disorders: An update. Curr Opin Neurol 35(2):220–229
Norcliffe-Kaufmann L, Kaufmann H, Palma JA, Shibao CA, Biaggioni I, Peltier AC et al (2018) Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Ann Neurol 83(3):522–531
Oertel WH, Henrich MT, Janzen A, Geibl FF (2019) The locus coeruleus: Another vulnerability target in Parkinson’s disease. Mov Disord 34(10):1423–1429
Ohtsuka C, Sasaki M, Konno K, Kato K, Takahashi J, Yamashita F et al (2014) Differentiation of early-stage parkinsonisms using neuromelanin-sensitive magnetic resonance imaging. Parkinsonism Relat Disord 20(7):755–760
Paredes-Rodriguez E, Vegas-Suarez S, Morera-Herreras T, De Deurwaerdere P, Miguelez C (2020) The Noradrenergic System in Parkinson’s Disease. Front Pharmacol 11:435
Poe GR, Foote S, Eschenko O, Johansen JP, Bouret S, Aston-Jones G et al (2020) Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci 21(11):644–659
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30(12):1591–1601
Prasuhn J, Prasuhn M, Fellbrich A, Strautz R, Lemmer F, Dreischmeier S et al (2021) Association of locus coeruleus and substantia nigra pathology with cognitive and motor functions in patients with Parkinson disease. Neurology 97:e1007–e1016
Remy P, Doder M, Lees A, Turjanski N, Brooks D (2005) Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 128(6):1314–1322
Samuels ER, Szabadi E (2008) Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol 6(3):235–253
Sasaki M, Shibata E, Tohyama K, Takahashi J, Otsuka K, Tsuchiya K et al (2006) Neuromelanin magnetic resonance imaging of locus coeruleus and substantia nigra in Parkinson’s disease. NeuroReport 17(11):1215–1218
Schwarz ST, Xing Y, Tomar P, Bajaj N, Auer DP (2017) In Vivo Assessment of Brainstem Depigmentation in Parkinson Disease: Potential as a Severity Marker for Multicenter Studies. Radiology 283(3):789–798
Solopchuk O, Sebti M, Bouvy C, Benoit CE, Warlop T, Jeanjean A, Zénon A (2018) Locus Coeruleus atrophy doesn’t relate to fatigue in Parkinson’s disease. Sci Rep 8(1):12381
Sommerauer M, Fedorova TD, Hansen AK, Knudsen K, Otto M, Jeppesen J et al (2018) Evaluation of the noradrenergic system in Parkinson’s disease: an 11C-MeNER PET and neuromelanin MRI study. Brain 141(2):496–504
Sun MK (1995) Central neural organization and control of sym- pathetic nervous system in mammals. Prog Neurobiol 47:157–233
Tong Q, Chen L (2021) Lack of Association of Locus Coeruleus Pathology with Orthostatic Hypotension in Parkinson’s Disease. J Parkinsons Dis 11(1):233–237
Van Bockstaele EJ, Pieribone VA, Aston-Jones G (1989) Diverse afferents converge on the nucleus paragigantocellularis in the rat ventrolateral medulla: retrograde and anterograde tracing studies. J Comp Neurol 290(4):561–584
Velseboer DC, de Haan RJ, Wieling W, Goldstein DS, de Bie RM (2011) Prevalence of orthostatic hypotension in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 17(10):724–729
Wang J, Li Y, Huang Z, Wan W, Zhang Y, Wang C et al (2018) Neuromelanin-sensitive magnetic resonance imaging features of the substantia nigra and locus coeruleus in de novo Parkinson’s disease and its phenotypes. Eur J Neurol 25(7):949-e73
Ye R, O’Callaghan C, Rua C, Hezemans FH, Holland N, Malpetti M et al (2022) Locus Coeruleus Integrity from 7 T MRI Relates to Apathy and Cognition in Parkinsonian Disorders. Mov Disord 37(8):1663–1672
Acknowledgements
The authors are grateful to the study participants.
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. Partly funded by Italian Ministry of Health (code: RF 2013, #PE2013-02359574[P.I.: F.S.G.]).
Author information
Authors and Affiliations
Contributions
GP and RC conceptualized the study; GP, GB, FL, DP, SDC, RC and FSG designed the methodology; GP, AG, GB, NM, and RM involved in formal analysis and investigated the data; GP and GB wrote the original draft and prepared the manuscript; GP, AG, RC, and FSG wrote, reviewed, and edited the manuscript; FSG acquired part of funding; and GS, RC, and FSG supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
All authors do not have competing interests to disclose concerning the present article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Palermo, G., Galgani, A., Bellini, G. et al. Neurogenic orthostatic hypotension in Parkinson’s disease: is there a role for locus coeruleus magnetic resonance imaging?. J Neural Transm 131, 157–164 (2024). https://doi.org/10.1007/s00702-023-02721-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-023-02721-7