Abstract
The majority of Greek lignite fields and coal-fired power plants is accumulated in the centre of Western Macedonia in the Ptolemais-Amynteo region of Northern Greece (Ptolemaida basin). The corresponding environmental impact dominantly caused by the mining process itself but also due to the burning process of lignites needs to be considered. Therefore, this study aimed at assessing the potential of PAHs to trace the environmental impact of power plant and coal mining emissions on soil pollution in the Ptolemaida basin. Noteworthy, the study area exhibits some special features (e.g. geomorphological and meteorological conditions, well-defined emission sources) that allow to prove the suitability of PAHs as indicators for specific petrogenic contaminations. The different sets of PAH emission sources followed in this study (fly ashes, lignite dust, charred woody material) reflected the pollution as derived from the coal mining activities as well as agricultural measures. Different approaches from simple (total amount) to more complex fingerprinting (ring member classification; profiles of sulphur-/nitrogen- and oxygen-containing PACs) as well as the application of established specific PAH ratios revealed no significant correlation with the detected soil contamination or rather soil PAH profiles. However, the microscopic analyses contradicted this assumption, since relevant particle concentrations were evident for fly ash as well as lignite particles. Hence, a huge superimposition by a further PAH emission source is assumed, probably the exhaust fumes of car traffic. In general, the study demonstrated that the application of PAHs as source indicators in soil systems is very complex and needs a very thorough interpretation. Hence, the need of using additionally complementary parameters for an unambiguous emission source apportionment seems obvious.
Graphical abstract

Similar content being viewed by others
Introduction
Over the last decades lignite has been utilized worldwide as an important raw material, mainly for electric power production. In the past Greece produced up to 80% of the electrical power by coal-fired power plants and was the fifth largest world and the second largest European producer of lignite. The majority of Greek coal-fired power plants is accumulated in the Lignite Center of Western Macedonia in the Ptolemais-Amynteo region of Northern Greece (Ptolemaida basin). Over the last 50 years, it has contributed more than 60% of the electrical power generated in the country (Kolovos et al. 2002a). The required lignite has been exploited in four opencast mines (Amynteo, Kardia, Southern and Main fields) feeding correspondingly four power plants (Amynteo, Ptolemaida, Liptol and the Agios Dimitrios power plants) of which the Agios Dimitrios Power Plant is the largest in Greece (Kantiranis et al. 2004; Georgakopoulos 2003)
The geochemical characteristics (including their economic implications) of these Greek lignites have already been studied intensively (e.g. Georgakopoulos 1995, 2001; Kolovos et al. 2002a,b; Iordanidis et al. 2012). In addition, the corresponding environmental impact on this region dominantly caused by the mining process itself (dust production, severe changes in the landscape, drainage system and infrastructure) but also due to the burning process of lignites has been considered. Noteworthy, the combustion process generates not only large volumes of solid wastes (fly ash mainly, bottom ash and slags), but also relevant air emissions (Georgakopoulos 2002). In particular, airborne particles emitted by power plants (fly ash-derived particles) or by lignite open-pit mining are transporting associated pollutants, from which polycyclic aromatic compounds (PAHs) have already been studied in more detail not only in Greece (e.g. Arditsoglou et al. 2004; Kalaitzoglou et al. 2004), but also in Bulgaria, Serbia, India, China and further affected regions (e.g. Ruwei et al. 2013; Apostolova et al. 2021; Buha-Markovic et al. 2020; Kostova et al. 2023; Verma et al. 2015). PAHs are important organic contaminants due to several reasons. They belong to the group of environmentally highly stable compounds, the so-called persistent organic pollutants POPs. Further on, several isomers exhibit highly relevant (eco)toxicological properties, most prominent example is benzo(a)pyrene exhibiting a high cancerogenic potential. But the individual patterns of isomers and homologues of this comprehensive substance group are also used to distinguish emission sources, dominantly pyrogenic from petrogenic ones. This has been applied especially to aquatic sediments (rivers, coastal areas) as described in detail by Yunker et al. (2002) and also to aquatic ecosystems influenced by coal mining activities (e.g. Pies et al. 2008; Gerslova & Schwarzbauer 2014).
However, for characterizing the secondary emission pathway from airborne fly ash or lignite particles towards the soil systems, PAHs have been studied to a lower extent, although it is known that PAHs exhibit a substantial stability in the pedosphere, especially in coal-enriched soils (e.g. Achten et al. 2011). Therefore, this study aimed at assessing the potential of PAHs to trace the environmental impact of power plant and coal mining emissions on soil pollution in the Ptolemaida basin. Besides detailed organic-geochemical analyses, microscopical measurements have also been applied on a wider set of soil samples to obtain a comprehensive set of possible indicators for a complementary characterization of these specific airborne emissions.
Material and methods
Study area
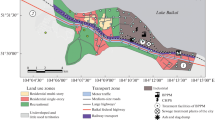
The Ptolemaida basin is situated in Northern Greece belonging to the West Macedonia. It is a ditch-like structure trending NW–SE and the average altitude is 700 m asl. The basin is bordered to the north by the lakes of Vegoritis, Petron and Chimaditis, to the west by the Askio mountain range with elevations up to 2111 m asl. and to the east by the Vérmio mountain range with elevations up to 2027 m asl. The hills of Drepano-Kozani comprise the southern boundary of the basin (see Fig. 1).
Sample material
This study compares sample material from selected emission sources with potentially affected soil samples (Table 1). For characterizing coal-related emissions, a lignite sample from the Ptolemaida open-pit mining area and four fly ash samples from the associated lignite power plants have been collected. Fly ash samples were taken either directly from the electrostatic precipitators of the furnace-units of the power plant or in the hoppers of the electrostatic precipitators. The fly ash samples were composed from a 30-day collection period.
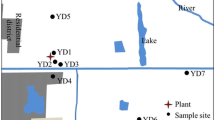
In total, 19 soil samples from the Ptolemaida basin have been collected comprising farmland soils, natural soils and one sample of an agricultural soil which has just been affected by a slash-and-burn treatment (El), a still common practice in Greece (Table 1). Samples were taken from the upper 10 cm and without any plant residues. The sampling locations are given in Fig. 1.
All samples were filled in precleaned glass vessels and stored in the dark at + 4°C until extraction.
Loss on ignition
As a basic bulk parameter, the loss-on-ignition LOI has been determined by heating aliquots of pre-dried samples at 550° in the presence of oxygen till constant weight. LOI values have been calculated by the difference of weight prior and after the treatment in relation to the initial amount.
Extraction and fractionation
Aliquots of 100 g of soil and fly ash samples but 10 g of the lignite sample were extracted sequentially by ultrasonic treatment. For the first extraction step, 50 mL of acetone were used and the ultrasonic treatment was applied for 15 min. After separation of the liquid phase by centrifugation at 1300 rpm, this extraction procedure was repeated with 100 mL of hexane. Thereafter, the organic phases were combined, the water phase was separated, and the raw extract was concentrated by rotary evaporation to a volume of approx. 5 mL. This solution was dried over anhydrous sodium sulphate and desulphurized by using activated copper powder.
After volume reduction down to 0.5 mL, the raw extract was fractionated by liquid chromatography on a micro-silica column (particle size: 40 µm, pore size: 60 Å, Baker) packed with 2 g of activated silica. The fractionation was carried out as previously described in more detail (Schwarzbauer et al. 2000). Briefly six fractions with increasing polarity were obtained.
To each of the non to medium polar fractions (fractions 1–5) 50 µL of a surrogate standard (containing 6.0 ng µL−1 of d34-hexadecane) was added. The acidic fraction (fraction 6) was derivatized by methylation with diazomethane as described previously (Kronimus et al. 2004) and 200 µL of a different surrogate standard (fluoroacetophenone with a concentration of 3.7 ng µL−1) was added. Prior to the following GC/MS analyses, the fractions were reduced to a final volume of approx. 50 µL.
Gas chromatographic/ mass spectrometric analyses GC/MS
Gas chromatographic/mass spectrometric analyses have been performed on a quadrupole mass spectrometer (Trace MS, Finnigan) linked with a gas chromatograph (GC5160, Carlo Erba). Gas chromatic separation was carried out on a capillary column (ZB5, Zebron, 25 m length × 0.25 mm ID × 0.25 µm film thickness). Injection type was a hot split/splitless injection (injector temperature 270 °C, 1 min splitless time) with an injection volume of approx. 1 µL. Temperature program was set to: 3 min isothermal time at 80 °C, heating from 80° to 310 °C with a heating rate of 5 K/min, and a final isothermal time of 15 min at 310 °C.
The mass spectrometer operated in the low-resolution EI+ mode with a scan time of 1 s of and a source temperature of 200 °C. The carrier gas helium was set to a flow of approx. 45 cm/min.
Identification of compounds was based on comparison (i) of mass spectra with those of libraries (NIST2014, Wiley 7th Ed) (ii) as well as of mass spectral and gas chromatographic properties with those of reference material. Quantitation was carried by peak integration in specific ion chromatograms and with an external calibration generated with authentic reference material. Inaccuracies of injection volume, etc., are corrected by the surrogate standard, recovery rates have been used for calculating final values.
Quality assessment of organic-geochemical analyses
For assessing the analytical quality, blank analyses, recovery rates and sensitivity have been evaluated. Blanks have been processed by executing the whole analytical procedure on a precleaned sand sample. GC/MS analyses revealed no appearance of the relevant compounds in the blanks.
The determination of recovery rates has been performed on precleaned soil material spiked with reference substances using amounts on the level of detected concentrations. Subsequently, the spiked material was subjected to extraction, fractionation and GC/MS analyses as described above. The obtained recovery rates ranged between 40% (e.g. naphthalene) and 91% (e.g. carbazole). These rates have been considered during calculating the quantitative results.
Sensitivity was determined based on signal-to-noise ratios in real sample analyses. For all components their limit of quantitation (LOQ) was below 0.1 ng/g. Hence, no attempts were made to quantify below this threshold. Compounds that were detectable (signal-to-noise ratio above 3) but with concentration below LOQ were marked as ‘ < LOQ’ (see Table S1).
Microscopical analyses
Microscopic investigations have been carried out on a limited set of samples. The samples were embedded in epoxy resin and polished. Microscopic analyses were performed using a Zeiss microscope (Microscope Axioplan with objective Zeiss Epiplan-Neofluar 50x/ 1.0 Oil Pol). The organic matter was identified and characterized using white light in incident light mode.
The microscopical quantitation was carried out by a point counting method as described by Taylor et al. (1998). The particle concentration of various carbonaceous particles has been expressed relative to the total volume of organic and inorganic matter (vol.%) (Table 2).
Results
Screening results
The application of GC/MS analyses on soil samples and potential petrogenic emission sources as a non-target screening approach revealed a broad spectrum of organic contaminants as summarized in Table 3 (and in extended version in Table S1 and S2). A first group of compounds identified solely in soil samples comprises chlorinated pesticides and derivatives. Besides already banned substances like DDT metabolites, lindane or hexachlorobenzene also more modern pesticides have been detected such as chlorpyrifos or dieldrin. Although the concentrations (see Table S1) are on a lower level of up to approx. 200 ng/g, the detection frequency of DDT metabolites is in particular remarkable. Also their occurrence in relation to the soil type is astonishing. Agricultural soils are either not contaminated (Pd) or only on low (El) to medium (Fs, Ko) levels. However, highest values have been detected in soils located nearby agricultural areas (Km, AD1). A clear attribution of pesticides as anthropogenic indicators for agricultural emissions did obviously not apply in the Ptolemaida region.
A ubiquitous group of contaminants were alkylated benzenes with methyl to C5 substitution. They were detected in nearly all soils samples (with exception of Zn) at concentrations of up to 190 ng/g. Alkylated benzenes appeared with similar concentrations also in fly ash as well as lignite samples.
However, a much higher structural variety and higher concentration levels were observed for polycyclic aromatic compounds (PACs) comprising polycyclic aromatic hydrocarbons (PAHs) but also their sulphur-/nitrogen-/oxygen-containing derivatives. The group of PAHs is clearly dominated by the parent compounds. For one representative, benz[e]pyrene, the maximum value of 3900 ng/g has been detected in soil AD2. Methylated and dimethylated isomers, e.g. of naphthalene, phenanthrene/anthracene or fluoranthene, were detected in similar concentration ranges as compared to their parent PAHs. The sulphur-/nitrogen- or oxygen-containing compounds appeared with clearly lower concentration levels as compared to the pure hydrocarbons. Within these PAC groups, the sulphur-containing substances dominated the two other groups.
PACs were also detected in fly ash and lignite samples, but with a different pattern. In the lignite sample the PAHs were detected in concentrations similar to higher contaminated soil samples whereas fly ash samples exhibited dominantly naphthalene and derivatives, but also higher PAHs to a very low extent. For the fossil-derived material, the sulphur-/nitrogen- or oxygen-containing compounds were either not detected (lignite, FA3) or appeared at very low concentration levels.
Particle characterization
By applying microscopical analyses, different types of particles have been examined pointing to the proposed emission from lignite mining and combustion. A principal differentiation between lignite particles and fly ash particles is based on their microscopical properties as illustrated in Fig. 2. Oxidation processes affect the chemical composition of lignite and, consequently, their optical properties, which is the clue to identify fly ash particles. A detailed description of the microscopical properties of fly ash particles has been reported by Iordanidis et al. (2008).
Further on, microscopical analyses also allow to characterize complementary pollution sources with potential to interfere with the detection of lignite-derived contamination. This accounts especially for burning of biomass, e.g. by slash-and -burn treatments in agriculture forming char coal particles.
Consequently, in this study three different types of particles are quantified in a preselected sample set, lignite and oxidized lignite particles (as fly ash representatives) as well as charred woody particles. Noteworthy, this applied microscopic method did not allow to detect air emissions released by traffic. Related very small particles are expected to have spherical shapes (aerosols with diameters of about 0.01 µm).
The results are summarized in Table 4. Lignite has certainly not been reported here since it represents one particle type. For fly ashes the specificity of oxidized lignite particles is fully confirmed, here only these particles have been identified to a relatively high amount of around 10 vol. %. In soil samples all three different particle types have been detected at various compositions and amounts. Oxidized lignite particles have been detected in all soil samples, whereas charred woody particles have been detected in only four samples but with enhanced amounts in the two agricultural soils analysed. Especially in sample EI, directly affected by a slash-and-burn treatment, the particle concentration reached 6.4 vol. %. Therefore, a direct linkage of the agricultural activity as emission source to the detected type of particle became evident.
Discussion
The study area exhibits some special features that allow to prove the suitability of PAHs as indicators for specific petrogenic contaminations. Firstly, the cities of Ptolemaida and Kozani are located in this area of extensive mining and combustion of lignites with different types of lignite-derived airborne contamination (lignite dust, fly ash from lignite power plants). Secondly, the geomorphologic situation and meteorological conditions in the basin favour a trapping of dust. Consequently, the particle-associated pollution remains concentrated in this area and the exchange as well as mixing of local particle associated pollution with those from more distant sources is limited. Especially during the winter season, the area faces a high frequency of low-speed winds and a strong temperature inversion and, consequently, the air pollutants are trapped (Voutsa 2004; Mavridou 2003). And thirdly, further different alternative PAH emission sources are identified here, predominantly car traffic as well as some agricultural practices, in particular the slash-and-burn treatment of harvested fields in the agricultural area around the mining sites.
As already introduced, for evaluating the suitability of PAHs to trace potential soil contamination by lignite mining and lignite-fed power plants different approaches from very coarse correlation to more specific parameters can be followed. A very simple approach is to compare the absolute contamination load by PAHs as illustrated in Fig. 3. In this study, highest absolute values have been measured in one soil sample followed by the lignite (both around 10 µg/g), whereas all further soil samples have clearly lower levels of up to ca. 0.2 µg/g. The fly ash samples exhibited very low levels which might be the result of the combustion processes. Noteworthy, some studies reported similar concentration levels (e.g. Apostolova et al. 2021, Kostova et al. 2023), but other investigations revealed higher PAH levels in fly ash (Arditsoglou et al. 2004; Buha-Markovic et al. 2020).
Total amounts of PAHs given as total amounts in ng/g (a) and normalized to loss-on-ignition as ng/g LOI (b). Sum of PAHs is calculated based on the values from Tab. S1
However, as important aspect the heterogeneity of the samples needs to be considered. Here, especially the organic matter content varies highly (LOI values from < 1% to 74%). Normalizing the PAH content by LOI as given in Fig. 3B reveals a slightly different pattern. Consequently, this simple parameter does not point to different pollution sources and remains unspecific.
More specifically, the coarse individual composition of PACs can be used for appointing emission sources. A simple approach is based on the differentiation of PAHs and sulphur-/ oxygen- and nitrogen-containing PACs (S-PACs, O-PACs, N-PACs) as illustrated in Fig. 4. The reference samples of lignite and fly ashes exhibit a unique pattern. PACs in the lignite samples are more or less exclusively composed by PAHs, whereas in most of the fly ash samples a significant proportion of S-PACs (but no N- or O-PACs) is visible. For the soil samples, a more variable pattern has been detected. Dominated by the pure hydrocarbons a remarkable variation is obvious for the PAC subfractions. All three hetero PAC subfractions have been detected, but without a stringent composition. Comparing the soil pattern with the lignite and fly ash pattern, no clear attribution of PAC contamination to these two emission sources is possible. Only the S-PAC value might have a slightly indicative character.
As a second, more precise parameter the PAH composition can be evaluated according to the aromatic ring numbers (e.g. Navarro-Ortega 2012). For this study, it is given in Fig. 4 as relative compositions. All two- to-five ring PAHs have been detected in lignite as well as soil samples with highly varying compositions. Noteworthy, in fly ash samples naphthalene and its derivatives (2-ring PAH) are clearly dominating. This might be the effect of the combustion process affecting intensively higher molecular weight organic compounds. Such shift has been also observed by Apostolova et al. (2021). However, the observed composition is contradictory to some other studies, in which the 3- and 4-ring PAHs dominated the PAH composition in fly ashes (e.g. Arditsoglou et al. 2004; Buha-Markovic et al. 2020). Interestingly, Ruwei et al. (2013) also reported a main amount of 5- to 6-ring PAH in bottom ashes, whereas a dominance of low-molecular weight PAHs was found in corresponding fly ashes. The opposite situation for bottom and fly ashes was observed by Verma a et al. (2015).
Here, the pattern of sample FA1 is remarkable that has a pronounced contribution of 5-ring and 6-ring PAHs pointing to an incomplete combustion probably due to technical malfunction. This assumption is supported by the LOI-normalized total amount measured for this sample (see Fig. 3B) which is much higher as compared to the other fly ash samples. From an environmental point of view, probably not the regular process in combustion power plants but malfunctions contribute significantly to the pollution by fly ash-derived particles. This aspect needs to be considered in linking specific markers to this emission source.
Since also the two more detailed PAC-/PAH-parameters did not specifically point to individual emission sources related to lignite and lignite combustion, the patterns of individual compounds need to be evaluated. An impression of the variety of such pattern is exemplified in Fig. 5. Also here, a high variety is evident and no clear attribution of the pattern to pollution sources is obviously possible. However, the differences between sample FA1 and FA3 point once again to an incomplete combustion in case of fly ash FA1.
Besides statistical methods (e.g. Burns et al. 1997; Zhang et al. 2008; Pies et al. 2008; Navarro-Ortega et al. 2012), one approach to differentiate emission sources based on PAH patterns has been successfully applied, in particular by Yunker et al. (2002). This approach is based on the different preferences of isomers during PAH formation by pyrolysis or fossil matter generation. Roughly, pyrogenic and petrogenic PAHs can be differentiated by specific ratios contrasting PAH isomers dominantly formed (i) by pyrolysis/combustion processes as the result of their higher thermodynamical stability (e.g. phenanthrene) from those formed; (ii) by diagenetic and catagenetic processes in sedimentary systems with predetermined cyclic structures (e.g. chrysene). This approach has already been applied in environmental assessments in regions with lignite mining and lignite combustion emissions, e.g. in the Czech Republic (e.g. Gerslova & Schwarzbauer 2014).
For the Ptolemaida region, the impact of the lignite industry has been tried to figure out by correlations of three different ratios as illustrated in Fig. 6. The ratios using fluoranthene (Fl), pyrene (Py), benzo(a)anthracene (BaA) and chrysene (Chr) have been selected due to their widespread usage in environmental studies (e.g. Pies et al. 2008; Tobiszewski & Namiesnik 2012). The specificity of the individual ratios differs forming the different zones of emission types in the graph (petroleum, combustion of wood/grass/coal, petroleum combustion …).
Indicative PAH ratios for differentiating pyrogenic from petrogenic sources according to Yunker et al. (2002)
A deeper look on the distribution of the samples analysed in this study revealed interesting implications. Noteworthy, for fly ash samples the parameters were applicable due to the lack of the compounds in these samples with exception of sample FA1. Here, as a result of the incomplete combustion and the corresponding substance spectrum, the parameter point to (coal) combustion. Hence, pollution by fly ashes based on PAH analyses remains neglected as long as the power plants work sufficiently.
More generally, the two parameters (Fl/Fl + Py and BaA/BaA + Chr) are partly contradictory. The Fl/Fl + Py ratio appoints most of the soil samples to a dominant impact by pyrogenic pollution, whereas the BaA/BaA + Chr ratio divides this sample set into different groups belonging to pyrogenic or petrogenic or mixed contaminations. Interestingly, the fly ash sample FA1 is clearly attributed to combustion, but the lignite sample is characterized also by pyrogenic contamination. Although the results are not distinct, a more dominant contribution of pyrogenic sources to airborne pollution in soils of this region is obvious by the PAH ratio approach. Nevertheless, the microscopic analyses clearly point to a contamination of most of the analysed soils by lignite and fly ash particles (see Table 4). Therefore, it seems that the petrogenic PAH pollution in these soils is heavily superimposed by pyrogenic sources. Here, the pollution by car and truck traffic might be a highly relevant aspect that cannot be validated by the analyses of this study.
Conclusions
This study aimed at assessing the potential of PAHs as marker compounds for coal emission in soils in a wider aspect. The different sets of PAH emission sources followed in this study (fly ashes, lignite dust, charred woody material) reflected the pollution as derived from the coal mining activities as well as agricultural measures. Different approaches from simple to more complex fingerprinting as well as the application of established specific PAH ratios revealed no significant correlation with the detected soil contamination or rather soil PAH profiles. This would imply a very low soil contamination level by fly ash or lignite dust in this area. However, the microscopic analyses contradicted this assumption, since relevant particle concentrations were evident for fly ash as well as lignite particles. Both observations might point to an enormous superimposition of coal-derived pollution (in a wider sense) by another PAH emission source, here probably the exhaust gases from car traffic. A special aspect needs to be considered in case of fly ash contamination. This type of particle pollution is only visible by organic-geochemical parameters in case of incomplete combustion processes, e.g. during power plant malfunctions.
More generally, the results of this study demonstrate that although PAHs represent an important group of environmental pollutants, the application of PAHs as source indicators in soil systems is very complex and needs a very thorough interpretation. Hence, the need of using complementary parameters for an unambiguous emission source apportionment seems obvious.
References
Achten C, Sheng S, Straub KL, Hofmann (2011) The lack of microbial degradation of polycyclic aromatic hydrocarbons from coal-rich soils. Environ Pollut 159:623-629
Apostolova D, Kostava I, Bechtel A, Stefanova M (2021) PAHs in feed coals and fly ashes from coal-fired thermal power plants in Bulgaria. Int J Coal Geol 243:103782
Arditsoglou A, Petaloti C, Terzi E, Sofoniou M, Samara C (2004) Size distribution of trace elements and polycyclic aromatic hydrocarbons in fly ashes generated in Greek lignite-fired power plants. Sci Total Environ 323:153–167
Burns WA, Mankiewicz PJ, Bence AE, Page DS, Parker KR (1997) A principal-component and least-squares method for allocating polycyclic aromatic hydrocarbons in sediment to multiple sources. Environ Toxicol Chem 16:1119–1131
Buha-Markovic JZ, Marinkovic AD, Nemoda SD, Savic JZ (2020) Distribution of PAHs in coal ashes from the thermal power plant and fluidized bed combustion system; estimation of environmental risk of ash disposal. Environ Pollut 266:115282
Georgakopoulos A, Fernández-Turiel JL, Filippidis A, Llorens JF, Kassoli-Fournaraki A, Querol X, Lopez-Soler A (1995) Trace element contents of the Lava xylite/ lignite and Ptolemais lignite deposits, Macedonia County, Greece. In: Proceedings of the 8th International Conference on Coal Science, Oviedo, edited by Pajares, J. A., and Tascón, J. M. D., Coal Sci Technol, Elsevier, Amsterdam, The Netherlands, 24:163 – 166.
Georgakopoulos A (2001) Trace elements in the Lava Xylite/ Lignite Deposit. Servia Basin Northern Greece Energy Sources 23:143
Georgakopoulos A, Filippidis A, Kassoli-Fournaraki A, Iordanidis A, Fernández-Turiel JL, Llorens JF, Gimeno D (2002) Environmentally important elements in fly ashes and their leachates of the power stations of Greece. Energy Sources 24:83–91
Geršlova E, Schwarzbauer J (2014) Hydrocarbon based indicators for characterizing potential sources of coal derived pollution in the vicinity of the Ostrava city. Environ Earth Sci 71:3211–3222
Iordanidis A, Buckmann J, Triantafyllou AG, Asvesta A (2008) Fly ash-airborne particles from Ptolemais-Kozani area, norther Greece, as determined by ESEM–EDX. Int J Coal Geol 73:63–73
Iordanidis A, Schwarzbauer J, Georgakopoulos A, van Lagen B (2012) Organic geochemistry of Amynteo lignite deposit, northern Greece: a multi-analytical approach. Geochem Int 50:159–178
Kalaitzoglou M, Terzi E, Samara C (2004) Patterns and sources of particle-phase aliphatic and polycyclic aromatic hydrocarbons in urban and rural sites of western Greece. Atmospheric Environ 38:2545–2560
Kolovos N, Georgakopoulos A, Filippidis A, Kavouridis C (2002a) The effects on the mined lignite quality characteristics by the intercalated thin layers of carbonates in Ptolemais mines. Northern Greece, Energy Sources 24:761–772
Kolovos N, Georgakopoulos A, Filippidis A, Kavouridis C (2002b) Utilization of lignite reserves and simultaneous improvement of dust emissions and operation efficiency of a power plant by controlling the calcium (total and free) content of the fed lignite. Application on the Agios Dimitrios Power Plant, Ptolemais. Greece Energy & Fuels 16:1516–1522
Kostava I, Apostolova D, Bechtel A, Groß D, Stefanova (2023) Fly ashes generated from coal-fired thermoelectric power plants on the Balkanian peninsula—Organic geochemical study. Int J Coal Geol, in press, 104326.
Kronimus A, Schwarzbauer J, Dsikowitzky L, Heim S, Littke R (2004) Anthropogenic organic contaminants in sediments of the Lippe river, Germany. Wat Res 38:3473–3484
Mavridou E, Antoniadis P, Khanaqa P, Riegel W, Gentzis T (2003) Paleoenvironmental interpretation of the Amynteon-Ptolemaida lignite deposit in northern Greece based on its petrographic composition. Int J Coal Geol 56:253–268
Navarro-Ortega A, Ratola N, Hildebrandt A, Alves A, Laeorte S, Barcelo D (2012) Environmental distribution of PAHs in pine needles, soils, and sediments. Environ Sci Pollut Res 19:677–688
Pies C, Hoffmann B, Petrowsky J, Yang Y, Ternes TA, Hofmann T (2008) Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in riverbank soils. Chemosphere 72:1594–1601
Ruwei W, Jiamei Z, Jingjing L, Liu G (2013) Levels and patterns of polycyclic aromatic hydrocarbons in coal-fired power plant bottom ash and fly ash from Huainan, China. Arch Environ Contam Toxicol 65193–202.
Taylor GH, Teichmüller M, Davis A, Diessel CFK, Littke R, Robert P (1998) Organic Petrology, Gebrüder Bornträger Berlin.
Schwarzbauer J, Littke R, Weigelt V (2000) Identification of specific organic contaminants for estimating the contribution of the Elbe river to the pollution of the German Bight. Org Geochem 31:1713–1731
Tobiszewski M, Namiesnik J (2012) PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut 162:110–119
Verma SK, Masto RE, Gautam S, Choudhury DP, Ram LC, Maiti SK, Maity S (2015) Investigations on PAHs and trace elements in coal and its combustion residues from a power plant. Fuel 162:138–147
Voutsa D, Terz H, Muller L, Samara C, Koumitzis T (2004) Profile analysis of organic micropollutants in the environment of a coal burning area, NW Greece. Chemosphere 55:595–604
Yunker MB, MacDonald RW, Roxanne V, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 34:1525–1541
Zhang W, Thang S, Wan C, Yue D, Ye Y, Wang X (2008) Source diagnostic of polycyclic aromatic hydrocarbons in urban runoff, dust, rain and canopy throughfall. Environ Pollut 153:594–601
Acknowledgements
The substantial support and help by Prof. Andreas Georgakopolous (Aristotle University of Thessaloniki) and Prof. Andreas Iordanidis (University of Western Macedonia) are highly appreciated. Financial support by the Ykida program (DAAD) is greatly acknowledged.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schwarzbauer, J., Vossen, J. The potential of polycyclic aromatic compounds as indicators for soil contamination by open-pit mining and utilization of lignite: a case study at Ptolemaida basin, Greece. Int J Earth Sci (Geol Rundsch) (2023). https://doi.org/10.1007/s00531-023-02363-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00531-023-02363-w