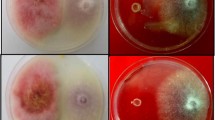
Abstract
Endophytic fungi live within plant tissues without harming the host, promote its growth, and protect host plants from abiotic and biotic stresses using different strategies. The present study aims to assess the antagonism of apple endophytic fungi with biocontrol potential on apple scab disease to control Botrytis cinerea and Macrophomina phaseolina in vitro and in vivo on tomato and melon plants. To this end, twelve endophyte isolates were evaluated in a dual culture test to control pathogen mycelia growth. The antifungal properties of the isolates were examined against B. cinerea and M. phaseolina in vitro to determine the release of volatile organic compounds, cellulase, and chitinase as antifungal mechanisms, as well as generating phosphate solubilization as growth-promoting effect. Based on the results, five isolates including Chaetomium globosum 2S1, C. globosum 3L2, Fusarium acuminatum GO2L1, Fusarium fujikuroi 37F6, and Fusarium incarnatum 25S3 were selected for further investigations. The results indicated that endophyte isolates C. globosum 2S1, F. acuminatum GO2L1, and F. fujikuroi 37F6 reduced the disease severity of B. cinerea isolates B1 and B2 by more than 80 and 70% on endophytically-colonized tomato, respectively. Different results were obtained from melon with disease control between 10 and 50%. The in vivo tests revealed the complete control of charcoal rot disease by C. globosum 2S1, C. globosum 3L2, F. acuminatum GO2L1, and F. incarnatum 25S3 on both endophytically colonized tomato and melon plants, unlike grey mold disease. Finally, the results indicated that endophytic isolate treatments did not significantly influence plant growth.



Similar content being viewed by others
References
Abro MA, Sun X, Li X, Jatoi GH, Guo LD (2019) Biocontrol potential of fungal endophytes against Fusarium oxysporum f. sp. cucumerinum causing wilt in cucumber. Plant Pathol J 35(6):598–608
Agrios GN (1997) Plant pathology. Academic Press, San Diego
Alabouvette C, Lemanceau P, Steinberg C (1993) Recent advances in biological control of Fusarium wilts. Pestic Sci 37:365–373
Alabouvette C, Olivain C, Migheli Q, Steinberg C (2009) Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt inducing Fusarium oxysporum. New Phytol 184:529–544
Alam B, Li J, Geˇ Q, Khan MA, Gong J, Mehmood S, Yuán Y, Gong W (2021) Endophytic fungi: from symbiosis to secondary metabolite communications or vice versa? Front Plant Sci 12:791033
Almeida F, Rodrigues ML, Coelho C (2019) The still underestimated problem of fungal diseases worldwide. Front Microbiol 10:214
Aly AH, Debbab A, Proksch P (2011) Fungal endophytes: unique plant inhabitants with great promises. Appl Microbiol Biotechnol 90(6):1829–1845
Azevedo JL (1998) Microrganismos endofíticos. Ecologia Microbiana. Jaguariúna. EMBRAPA 117:137
Bamisile BS, Dash CK, Akutse KS, Keppanan R, Wang LD (2018) Fungal endophytes: beyond herbivore management. Front Microbiol 9:11
Baron NC, Rigobelo EC (2022) Endophytic fungi: a tool for plant growth promotion and sustainable agriculture. Mycology 13(1):39–55
Berger LR, Reynolds DM (1958) The chitinase system of a strain of Streptomyces griseus. Biochim Biophy Acta 29:522–534
Boyle C, Guske S, Dammann U, Schulz B (1998) Endophyte host interactions. II. Defining symbiosis of the endophyte-host interaction. Symbiosis 25:213–227
Chen X, Wang Y, Gao Y, Gao T, Zhang D (2019) Inhibitory abilities of Bacillus isolates and their culture filtrates against the gray mold caused by Botrytis cinerea on postharvest fruit. Plant Pathol J 35(5):425–436
Dastogeer KM (2018) Influence of fungal endophytes on plant physiology is more pronounced under stress than well-watered conditions: a meta-analysis. Planta 248(6):1403–1416
del Castillo DS, Parra D, Noceda C, Perez-Martinez S (2016) Co-occurrence of pathogenic and non-pathogenic Fusarium decemcellulare and Lasiodiplodia theobromae isolates in cushion galls disease of cacao (Theobroma cacao L.). J Plant Prot Res 56(2):129–138
Dennis C, Webster J (1971) Antagonistic properties of specific groups of Trichoderma: production of non-volatile antibiotics. TBMS 57:25–39
Dhingra OD, Sinclair JB (1978) Biology and pathology of Macrophomina phaseolina. Minas Gerais: Universidade Federal de Viçosa: Impresa Universitaria
Dutta D, Puzari KC, Gogoi R, Dutta P (2014) Endophytes: exploitation as a tool in plant protection. Braz Arch Biol Technol 7(5):621–629
Ebrahimi L, Hatami Rad S, Ayenekar T, Agh-Atabay ME, Moghimi H, Etebarian HR (2021) New records of apple endophytic fungi for the Funga of Iran. Mycologia Iranica 8(2):31–39
Ebrahimi L, Hatami Rad S, Etebarian HR (2022) Apple endophytic fungi and their antagonism against apple scab disease. Front Microbiol 13:1024001
Etebarian HR, Kheiri A, Roustaei A, Khodakaramian GH, Aminian H (2007) Evaluation of Pseudomonas isolates for biological control of charcoal stem rot of melon caused by Macrophomina phaseolina. Acta Hortic 761:157–162 (In Persian)
Etebarian HR, Sholberg PL, Eastwell KC, Sayler RJ (2005) Biological control of apple blue mold with Pseudomonas fluorescens. Can J Microbiol 51:591–598
Ghosh T, Biswas MK, Guin C, Roy P (2018) A review on characterization, therapeutic approaches and pathogenesis of Macrophomina phaseolina. Plant Cell Biotechnol Mol Biol 19:72–84
Golafrouz H, Safaie N, Khelghatibana F (2020) The reaction of some apple rootstocks to biocontrol of white root rot Rosellinia necatrix by Trichoderma harzianum in greenhouse. J Crop Prot 9(4):577–589
González V, Armijos E, Garcés-Claver A (2020) Fungal endophytes as biocontrol agents against the main soil-borne diseases of melon and watermelon in Spain. Agron 10(6):820
Gouda S, Das G, Sem SK, Shin HS, Patra JK (2016) Endophytes: a treasure house of bioactive compounds of medicinal importance. Front Microbiol 7:1538
Govinda Rajulu MB, Thirunavukkarasu N, Suryanarayanan TS, Ravishankar JP, El Gueddari NE, Moerschbacher BM (2011) Chitinolytic enzymes from endophytic fungi. Fungal Divers 47:43–53
Gupta S, Chaturvedi P, Kulkarni MG, Staden JV (2018) A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol Adv 39:107462
Herrera-Téllez VI, Cruz-Olmedo AK, Plasencia J, Gavilanes-Ruíz M, Arce-Cervantes O, Hernández-León S, Saucedo-García M (2019) The protective effect of Trichoderma asperellum on tomato plants against Fusarium oxysporum and Botrytis cinerea diseases involves inhibition of reactive oxygen species production. Int J Mol Sci 20:2007
Hsu SC, Lockwood JL (1975) Powdered chitin agar as a selective medium for enumeration of Actinomycetes in water and soil. Appl Microbiol 29:422–426
Iida Y, Ogata A, Kanda H, Nishi O, Sushida H, Higashi Y, Tsuge T (2022) Biocontrol activity of nonpathogenic strains of Fusarium oxysporum: colonization on the root surface to overcome nutritional competition. Front Microbiol 13:826677
Jalali H, Ebrahimi L, Etebarian HR (2021) Biocontrol of tomato gray mold disease by Trichoderma harzianum and Bacillus subtilis. J Crop Prot 10(4):647–657
Jia M, Chen L, Xin HL, Zheng CJ, Rahman K, Han T, Qin LPA (2016) Friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front Microbiol 7:906
Jimenez-Diaz RM, Blanco-López MA, Sackston WE (1983) Incidence and distribution of charcoal rot of sunflower caused by Macrophomina phaseolina in Spain. Plant Dis 67(9):1033–1036
Kaur R, Kaur J, Singh RS (2010) Nonpathogenic Fusarium as a biological control agent. Plant Pathol J 9:79–91
Latz MAC, Jensen B, Collinge DB, Jørgensen HJL (2018) Endophytic fungi as biocontrol agents: elucidating mechanisms in disease suppression. Plant Ecol Divers 11:555–567
Lillbro M (2005) Biocontrol of Penicillium roqueforti on grain-acomparison of mode of action of several yeast species. Master thesis of the agriculture program, animal science, performed at the Department of Microbiology. Swedish University of Agricultural Sciences, 21 pp
Louvet J, Rouxel F, Alabouvette C (1976) Recherches sur la résistance des sols aux maladies. I. Mise en évidence de la nature microbiologique de la résistance d’un sol au développement de la fusariose vasculaire du melon. Ann Phytopathol 8:425–436
Majidi S, Roayaei M, Ghezelbash G (2011) Carboxymethyl-cellulase and filter-paperase activity of new strains isolated from Persian Gulf. Microbiol J 1:8–16
Marques NP, de Cassia Pereira J, Gomes E, da Silva R, Araújo AR, Ferreira H et al (2018) Cellulases and xylanases production by endophytic fungi by solid state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Ind Crop Prod 122:66–75. https://doi.org/10.1016/j.indcrop.2018.05.022
Marquez N, Giachero ML, Declerck S, Ducasse DA (2021) Macrophomina phaseolina: general characteristics of pathogenicity and methods of control. Front Plant Sci 12:634397
Meenavalli B, Rajulu G, Thirunavukkarasu N, Suryanarayanan TS, Ravishankar JP, Gueddari NEE, Moerschbacher BM (2011) Chitinolytic enzymes from endophytic fungi. Fungal Divers 47:43–53
Morais EM, Silva AAR, Sousa FWA, Azevedo IMB, Silva HF, Santos AMG, Júnior JEAB, de Carvalho CP, Eberlin MN, Porcari AM, da Silva Araújo FD (2022) Endophytic Trichoderma strains isolated from forest species of the Cerrado-Caatinga ecotone are potential biocontrol agents against crop pathogenic fungi. PLoS ONE 17(4):e0265824
Navarro-Meléndez AL, Heil M (2014) Symptomless endophytic fungi suppress endogenous levels of salicylic acid and interact with the jasmonate-dependent indirect defense traits of their host, lima bean (Phaseolus lunatus). J Chem Ecol 40:816–825
Olea AF, Bravo A, Martínez R, Thomas M, Sedan C, Espinoza L, Zambrano E, Carvajal D, Silva-Moreno E, Carrasco H (2019) Antifungal activity of eugenol derivatives against Botrytis cinerea. Molecules 24:1239
Peters S, Aust HJ, Draeger S, Schulz B (1998) Interactions in dual cultures of endophytic fungi with host and nonhost plant calli. Mycologia 90:360–367
Petrini O (1991) Fungal endophyte of tree leaves. In: Andrews JH, Hirano SS (eds) Microbial ecology of leaves, Brock/Springer Series in Contemporary Bioscience. Springer, New York, pp 179–197
Pimentel D (2009) Pesticides and pest control. In: Peshin R (ed) Integrated pest management: innovation-development process, vol 1. Springer, Netherlands, Dordrecht, pp 83–87
Rai M, Rathod D, Agarkar G, Dar M, Brestic M, Pastore GM, Marostica MRJ (2014) Fungal growth promotor endophytes: a pragmatic approach towards sustainable food and agriculture. Symbiosis 62:63–79
Rodriguez RJ, White JF, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182:314–330
Saikkonen K, Faeth SH, Helander M, Sullivan TJ (1998) Fungal endophytes: a continuum of interactions with host plants. Annu Rev Ecol Syst 29:319–343
Saito H, Sasaki M, Nonaka Y, Tanaka J, Tokunaga T, Kato A, Thuy TTT, Vang LV, Tuong LM, Kanematsu S, Suzuki T, Kurauchi K, Fujita N, Teraoka T, Komatsu K, Arie T (2021) Spray application of nonpathogenic fusaria onto rice flowers controls bakanae disease (caused by Fusarium fujikuroi) in the next plant generation. Appl Environ Microbiol 87:e01959–20
Saraiva RM, Czymmek KJ, Borges ÁV, Caires NP, Maffia LA (2015) Confocal microscopy study to understand Clonostachys rosea and Botrytis cinerea interactions in tomato plants. Biocontrol Sci Technol 25(1):56–71
Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A (2019) The global burden of pathogens and pests on major food crops. Nat Ecol Evol 3:430–439
Schulz B, Boyle C (2005) The endophytic continuum. Mycol Res 109:661–686
Schulz B, Boyle C, Draeger S, Römmert AK, Krohn K (2002) Review: endophytic fungi: a source of novel biologically active secondary metabolites. Mycol Res 106:996–1004
Schulz B, Haas S, Junker C, Andrée N, Schobert M (2015) Fungal endophytes are involved in multiple balanced antagonisms. Curr Sci 109(1):39–45
Schulz B, Römmert AK, Dammann U, Aust HJ, Strack D (1999) The endophyte-host interaction: a balanced antagonism. Mycol Res 103:1275–1283
Shirali A (2017) Isolation and identification of fungi associated with melon crown and root rot disease in south east of Tehran. MSc thesis in Plant pathology, University of Tehran
Shtienberg D, Elad Y, Niv A, Nitzani Y, Kirshner B (1998) Significance of leaf infection by Botrytis cinerea in stem rotting of tomatoes grown in non-heated greenhouses. Eur J Plant Pathol 104:753–763
Sperber JI (1958) The incidence of apatite-solubilizing organisms in the rhizosphere and soil. Crop Pasture Sci 9(6):778–781
Sridharan AP, Thankappan S, Karthikeyan G, Uthandi S (2020) Comprehensive profiling of the VOCs of Trichoderma longibrachiatum EF5 while interacting with Sclerotium rolfsii and Macrophomina phaseolina. Microbiol Res 236:126436
Steel RGD, Torrie JH (1980) Principles and procedures of statistic. McGraw Hill book Co Inc, New York
Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67(4):491–502
Suryanarayanan TS, Thirunavukkarasu N, Govinda Rajulu MB, Gopalan V (2012) Fungal endophytes: an untapped source of biocatalysts. Fungal Divers 54:19e30
Terhonen E, Blumenstein K, Kovalchuk A, Asiegbu FO (2019) Forest tree microbiomes and associated fungal endophytes: functional roles and impact on forest health. Forests 10:42
Toghueo RMK, Zabalgogeazcoa I, Vazquez de Aldana BR, Boyom FF (2017) Enzymatic activity of endophytic fungi from the medicinal plants Terminalia catappa, Terminalia mantaly and Cananga odorata. South Afr J Bot 109:146–153
Vimal SR, Singh JS, Arora NK, Singh S (2017) Soil-Plant-microbe interactions in stressed agriculture management: a review. Pedosphere 27:177–192
von Roepenack-Lahaye E, Boettcher C, Schulz B, Lahaye T, Rosahl S, Scheel D (2007) A metabolomics platform - transferring functional genomics technology from Arabidopsis to crop plants. In proceedings of the molecular plant-microbe interactions congress, Munich, Germany
Wilkins K, Nielsen KF, Din SU (2003) Patterns of volatile metabolites and nonvolatile Trichothecenes produced by isolates of Stachybotrys, Fusarium, Trichoderma, Trichothecium, and Memnoniella. Environ Sci Pollut Res 10:162–166
Yang SX, Gao JM, Zhang Q, Laatsch H (2011) Toxic polyketides produced by Fusarium sp., an endophytic fungus isolated from Melia azedarach. Bioorg Med Chem Lett 21:1887–1889
Yang SZ, Peng LT, Su XJ, Chen F, Cheng YJ, Fan G, Pan SY (2011) Bioassay guided isolation and identification of antifungal components from propolis against Penicillium italicum. Food Chem 127:210–215
Zhang Q, Zhang J, Yang L, Zhang L, Jiang D, Chen W, Li G (2014) Diversity and biocontrol potential of endophytic fungi in Brassica napus. Biol Control 72:98–108
Zikankuba VL, Mwanyika G, Ntwenya JE, James A (2019) Pesticide regulations and their malpractice implications on food and environment safety. Cogent Food Agric 5(1):15
Acknowledgements
We gratefully acknowledge the University of Tehran, Iran, for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Research involving human and/or animal rights
This article does not contain any studies with human participants or animals (vertebrates) performed by any of the authors.
Additional information
Handling Editor: Sotiris Tjamos
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ebrahimi, L., Tadayon Rad, F. & Lotfi, M. Antagonism of endophytic fungi depends on pathogen and host plant. BioControl 68, 655–668 (2023). https://doi.org/10.1007/s10526-023-10224-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-023-10224-3