Abstract
The repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) eliminates alkyl lesions that play the main anticancer role in alkylating chemotherapy. The inhibition of MGMT leads to increasing effectiveness of alkylating chemotherapy. In this study, new potential MGMT inhibitors were tested. It was found that some compounds demonstrate low cytotoxicity and high effectiveness in human cells in vitro.






Similar content being viewed by others
Change history
31 January 2024
Modifications have been made to the Publisher’s Note.
REFERENCES
Arslan, F.T., Yurdakok-Dikmen, B., Akgedik, R., and Topcu, G., Nitrosoguanidine-induced genotoxicity and oxidative stress in human gastric adenocarcinoma cells, Mutat. Res., 2016, vol. 797, pp. 28–34.
Chae, M.-Y., Swenn, K., Kanugula, S., Dolan, M.E., Pegg, A.E., and Moschel, R.C., 8-substituted O6-benzylguanine, substituted 6(4)-(benzyloxy)pyrimidine, and related derivatives as inactivators of human O6-alkylguanine-DNA alkyltransferase, J. Med. Chem., 1995, vol. 38, no. 2, pp. 359–365. https://doi.org/10.1021/jm00002a018
Christmann, M., Verbeek, B., Roos, W.P., and Kaina, B., O6-Methylguanine-DNA methyltransferase (MGMT) in normal tissues and tumors: Enzyme activity, promoter methylation and immunohistochemistry, Biochim. Biophys. Acta, Rev. Cancer, 2011, vol. 1816, no. 2, pp. 179–190. https://doi.org/10.1016/j.bbcan.2011.06.002
Dolan, M., Chae, M., Pegg, A., et al., Metabolism of O6-benzylguanine, an inactivator of O6-alkylguanine-DNA alkyltransferase, Cancer Res., 1994, vol. 54, no. 19, pp. 5123–5130. https://doi.org/10.1158/0008-5472.CAN-14-2047
Green, S.J. and Michael, R., Molecular Cloning, New York: Cold Spring Harbor Lab., 2012.
Griffin, R.J., Arris, C.E., Bleasdale, C., Boyle, F.T., Calvert, A.H., Curtin, N.J., Dalby, C., Kanugula, S., Lembicz, N.K., Newell, D.R., Pegg, A.E., and Golding, B.T., Resistance-Modifying Agents. 8. Inhibition of O 6-Alkylguanine-DNA Alkyltransferase by O 6-Alkenyl-, O 6-Cycloalkenyl-, and O 6-(2-Oxoalkyl)guanines and Potentiation of Temozolomide Cytotoxicity in Vitro by O6-(1-Cyclopentenylmethyl)guanine, J. Med. Chem., 2000, vol. 43, no. 22, pp. 4071–4083. https://doi.org/10.1021/jm000961o
Kaina, B., Margison, G.P., and Christmann, M., Targeting O 6-methylguanine-DNA methyltransferase with specific inhibitors as a strategy in cancer therapy, Cell. Mol. Life Sci., 2010, vol. 67, no. 21, pp. 3663–3681. https://doi.org/10.1007/s00018-010-0491-7
Kotsarenko, K.V., Lylo, V.V., Macewicz, L.L., Dasyukevich, O.I., Poltoratskaya, L.V., and Burkovskaya, T.E., Changes in the MGMT gene expression under the influence of exogenous cytokines in human cells in vitro, Cytol. Genet., 2013, vol. 47, no. 4, pp. 202–209. https://doi.org/10.3103/S0095452713040087
Kotsarenko, K., Lylo, V., Ruban, T., Macewicz, L., and Lukash, L., Effects of some growth factors and cytokines on the expression of the repair enzyme MGMT and protein MARP in human cells in vitro, Biochem. Genet., 2018, vol. 56, pp. 459–477. https://doi.org/10.1007/s10528-018-9854-9
Lipinski, C.A., Lead- and drug-like compounds: the rule-of-five revolution, Drug Discovery Today: Technol., 2004, vol. 1, no. 4, pp. 337–341. https://doi.org/10.1016/j.ddtec.2004.11.007
Lopez, S., Margison, G.P., McElhinney, R.S., Cordeiro, A., McMurry, T.B H., and Rozas, I., Towards more specific O 6-methylguanine-DNA methyltransferase (MGMT) inactivators, Bioorg. Med. Chem., 2011, vol. 19, no. 5, pp. 1658–1665. https://doi.org/10.1016/j.bmc.2011.01.038
Lukash, L.L., Bodt, J., Pegg, A.E., Dolan, M.E., Maher, V.M., and McCormick, J., Effect of O 6-alkylguanine-DNA alkyltransferase on the frequency and spectrum of mutations induced by N-methyl-N’-nitro-N-nitrosoguanidine in the HPRT gene of diploid human fibroblasts, Mutat. Res., 1991, vol. 250, no. 12, pp. 397–409. https://doi.org/10.1016/0027-5107(91)90196-U
McElhinney, R., Donnelly, D., McCormick, A., et al., Inactivation of O 6-alkylguanine-DNA alkyltransferase. 1. Novel O 6-(hetarylmethyl)guanines having basic rings in the side chain, J. Med. Chem., 1998, vol. 41, no. 26, pp. 5265–5271. https://doi.org/10.1021/jm9804388
Mitra, S., MGMT: A personal perspective, DNA Repair, 2007, vol. 6, no. 8, pp. 1064–1070. https://doi.org/10.1016/j.dnarep.2007.03.007
Mohanty, S., Sharma, P., Gupta, P.K., and Chatterjee, S., Nitrosoguanidine-induced DNA damage response in Escherichia coli cells, Mutat. Res., 2019, vol. 842, pp. 18–27. https://doi.org/10.1016/j.mrgentox.2019.04.006
Morris, G.M., Huey, R., Lindstrom, W., Sanner, M.F., Belew, R.K., Goodsell, D.S., and Olson, A.J., AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexiblity, J. Comput. Chem., 2009, vol. 30, pp. 2785–2791.
Moschel, R.C., McDougall, M., Dolan, M.E., Pegg, A.E., and Phillips, D.R., Structural features of substituted purine derivatives compatible with depletion of human O 6-alkylguanine-DNA alkyltransferase, J. Med. Chem., 1992, vol. 35, no. 23, pp. 4486–4491. https://doi.org/10.1021/jm00103a019
Oshiro, S., Tsugu, H., Komatsu, F., Ohmura, T., Ohta, M., Sakamoto, S., Fukushima, T., and Inoue, T., Efficacy of Temozolomide Treatment in Patients with High-grade Glioma, Anticancer Res., 2009, vol. 29, pp. 911–918. https://ar.iiarjournals.org/content/29/3/911.full.
Pauly, G., Loktionova, N., Fang, Q., et al., Substitution of aminomethyl at the meta-position enhances the inactivation of O 6-alkylguanine-DNA alkyltransferase by O 6-benzylguanine, J. Med. Chem., 2008, vol. 51, no. 22, pp. 7144–7153. https://doi.org/10.1021/jm800758y
Pedretti, A., Mazzolari, A., Gervasoni, S., Fumagalli, L., and Vistoli, G., The VEGA suite of programs: an versatile platform for cheminformatics and drug design projects, Bioinformatics, 2021, vol. 37, no. 8, pp. 1174–1175. https://doi.org/10.1093/bioinformatics/btaa774
Pegg, A.E., Repair of O 6-alkylguanine by alkyltransferases, Mutat. Res., 2000, vol. 462, nos. 2–3, pp. 83–100. https://doi.org/10.1016/s1383-5742(00)00017-x
Pegg, A.E., Multifaceted roles of alkyltransferase and related proteins in DNA Repair, DNA damage, resistance to chemotherapy, and research tools, Chem. Res. Toxicol., 2011, vol. 24, no. 5, pp. 618–639. https://doi.org/10.1021/tx200031q
Quinn, J.A., Jiang, S.X., Carter, J., Reardon, D.A., Desjardins, A., Vredenburgh, J.J., Friedman, H.S., Phase II trial of gliadel plus O 6-benzylguanine in Adults with recurrent glioblastoma multiforme, Clin. Cancer Res., 2009, vol. 15, no. 3, pp. 1064–1068. https://doi.org/10.1158/1078-0432.ccr-08-2130
Ranson, M., Lomeguatrib, a potent inhibitor of O 6-alkylguanine-DNA-alkyltransferase: Phase I safety, pharmacodynamic, and pharmacokinetic trial and evaluation in combination with temozolomide in patients with advanced solid tumors, Clin. Cancer Res., 2006, vol. 12, no. 5, pp. 1577–1584. https://doi.org/10.1158/1078-0432.ccr-05-2198
Ruiz, F., Gil-Redondo, R., Morreale, A., et al., Structure-based discovery of novel non-nucleosidic DNA alkyltransferase inhibitors: virtual screening and in vitro and in vivo activities, J. Chem. Inf. Modell., 2008, vol. 48, no. 10, pp. 1972–1982. https://doi.org/10.1021/ci800202t
Sharma, S., Salehi, S., Yang, Y., and Vessella, R.L., Role of MGMT in tumor development, progression, diagnosis, treatment and prognosis, Anticancer Res., 2009, vol. 29, no. 10, pp. 3759–3768. https://doi.org/10.1016/j.etap.2019.03.012
Terashima, I. and Kohda, K., Inhibition of human O 6-alkylguanine-DNA alkyltransferase and potentiation of the cytotoxicity of chloroethylnitrosourea by 4(6)-(benzyloxy)-2,6(4)-diamino-5-(nitro or nitroso)pyrimidine derivatives and analogues, J. Med. Chem., 1998, vol. 41, no. 4, pp. 503–508. https://doi.org/10.1021/jm970712f
Verbeek, B., Southgate, T.D., Gilham, D.E., and Margison, G.P., O 6-methylguanine-DNA methyltransferase inactivation and chemotherapy, Br. Med. Bull., 2008, vol. 85, no. 1, pp. 17–33. https://doi.org/10.1093/bmb/ldm036
Volynets, G.P., Ruban, T.P., Yatsyshina, A.P., Matsevich, L.L., Bdzhola, V.G., Yarmolyuk, S.M., and Lukash, L.L., RF Patent 127059, 2018. https:// base.uipv.org/searchINV/search.php?action=viewdetails&IdClaim=2.492.48.
Volynets, G.P., Yatsyshina, A.P., Ruban, T.P., Matse-vich, L.L., Nidoyeva, Z.M., Balanda, A.O., Bdzhola, V.G., Yarmolyuk, S.M., and Lukash, L.L., Ukraine Patent 122373, 2020. https://base.uipv.org/ searchINV/search.php?action=viewdetails&IdClaim =271905.
Wang, C., Abegg, D., Hoch, D., and Adibekian, A., Chemoproteomics-enabled discovery of a potent and selective inhibitor of the DNA repair protein MGMT, Angew. Chem., Int. Ed., 2016, vol. 55, no. 10, pp. 2911–2915. https://doi.org/10.1002/anie.201510203
Yu, W., Zhang, L., Wei, Q., and Shao, A., O6-methylguanine-DNA methyltransferase (MGMT): challenges and new opportunities in glioma chemotherapy, Front. Oncol., 2020, vol. 9, p. 1547. https://doi.org/10.3389/fonc.2019.01547
Zhang, X., Zhou, Y., Hu, Y., and Huang, P., Nitrosoguanidine-induced DNA damage and cell cycle arrest in human liver cells, Environ. Toxicol. Pharmacol., 2019, vol. 68, pp. 66–71. https://doi.org/10.1016/j.etap.2019.03.012
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
CONCLUSIONS
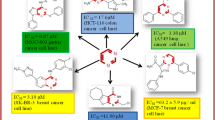
In this study we examined a number of compounds for their potential as MGMT inhibitors and assessed their cytotoxicity and effectiveness in vitro. Results indicated that compounds 41 (5-(5-Сhloro-2-hydroxy-benzylidene)-4-thioxo-thiazolidin-2-one), 41B (5-Benzo[1,3]dioxol-5-ylmethylene-thiazolidine-2,4-dione), and 89 (2-[5-(4-Bromo-phenyl)-pyrimidin-4-yl]-5-ethoxy-phenol) showed promising characteristics with lower cytotoxicity and higher effectiveness compared to the standard inhibitor BG at a concentration of 10 µM.
Additional information
Publisher’s Note.
Allerton Press remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Zhuvaka, K.S., Volynets, G.P., Ruban, T.P. et al. Activity of Nonnucleoside Inhibitors of O6-methylguanine-DNA Methyltransferase Repair Enzyme in Human Cells In Vitro. Cytol. Genet. 57, 556–566 (2023). https://doi.org/10.3103/S0095452723060105
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452723060105