Abstract
Purpose
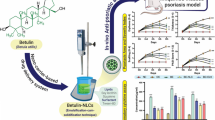
Psoriasis is a chronic autoimmune inflammatory cutaneous disorder, and single-drug therapy is inadequate for curing this disease. Dual-drug therapy with multi-target synergistic effects may be an alternative approach to eradicate psoriasis. This study reports the development of a lipid-polymer hybrid nanoparticle (LPHNP)-based polymeric hydrogel for topical delivery of methoxsalen (MS) and curcumin (CUR) for the management of psoriasis.
Methods
MS-CUR-LPHNPs were prepared using the emulsification solvent evaporation method and incorporated into a Carbopol-940-based polymeric hydrogel for topical application. The antipsoriatic efficacy of the hydrogel was evaluated in an imiquimod (IMQ)-induced psoriasis rat model.
Results
Methoxsalen-co-loaded curcumin lipid-polymer hybrid nanoparticles (MS-CUR-LPHNPs, 206.8 ± 3.2 nm) were successfully prepared with a narrow polydispersity index (PDI = 0.174), negative zeta potential (− 27.1 ± 6.09 mV), and entrapment efficiency of 84.90 ± 0.68%. The polymeric hydrogel showed all the desirable characteristics essential for topical application. The MS-CUR-LPHNP-based polymeric hydrogel achieved superior anti-psoriatic effects in the IMQ-induced psoriasis rat model because of the high dermal retention of dual drugs for an extended period compared to a standard marketed anti-psoriatic formulation.
Conclusion
Therefore, we concluded that the developed MS-CUR-LPHNPs (D6-HNPs) were novel, providing synergistic therapeutic efficacy and promising prospects for the management of psoriasis.












Similar content being viewed by others
Data Availability
Data available on request to the corresponding author.
References
Griffiths CEM, van der Walt JM, et al. The global state of psoriasis disease epidemiology: a workshop report. Br J Dermatol. 2017;177(1):e4–7.
Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475.
Saleem S, Iqubal MK, Garg S, et al. Trends in nanotechnology-based delivery systems for dermal targeting of drugs: an enticing approach to offset psoriasis. Expert Opin Drug Deliv. 2020;17(6):817–38.
Yamanaka K, Yamamoto O, Honda T. Pathophysiology of psoriasis: a review. J Dermatol. 2021;48(6):722–31.
Bakshi H, Nagpal M, Singh M, et al. Treatment of psoriasis: a comprehensive review of entire therapies. Curr Drug Saf. 2020;15(2):82–104.
Rapalli VK, Kaul V, Waghule T, et al. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur J Pharm Sci. 2020;152: 105438.
Yu Z, Meng X, Zhang S, et al. Recent progress in transdermal nanocarriers and their surface modifications. Molecules. 2021;26(11):3093.
Carter P, Narasimhan B, Wang Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery of various skin diseases. Int J Pharm. 2019;555:49–62.
Yu YQ, Yang X, Wu XF, et al. Enhancing permeation of drug molecules across the skin via delivery in nanocarriers: novel strategies for effective transdermal applications. Front Bioeng Biotechnol. 2021;9: 646554.
Kahraman E, Güngör S, Özsoy Y. Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery. Ther Deliv. 2017;8(11):967–85.
Mitchell MJ, Billingsley MM, Haley RM, et al. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discovery. 2021;20(2):101–24.
Chen ZJ, Yang SC, Liu XL, et al. Nanobowl-supported liposomes improve drug loading and delivery. Nano Lett. 2020;20(6):4177–87.
Kamaly N, Yameen B, Wu J, et al. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–63.
Zhuang Y, Zhao Y, Wang B, et al. Strategies for preparing different types of lipid polymer hybrid nanoparticles in targeted tumor therapy. Curr Pharm Des. 2021;27(19):2274–88.
Du X, Gao N, Song X. Bioadhesive polymer/lipid hybrid nanoparticles as oral delivery system of raloxifene with enhancive intestinal retention and bioavailability. Drug Deliv. 2021;28(1):252–60.
Fereig SA, El-Zaafarany GM, Arafa MG, et al. Self-assembled tacrolimus-loaded lecithin-chitosan hybrid nanoparticles for in vivo management of psoriasis. Int J Pharm. 2021;608: 121114.
Pukale SS, Mittal A, Chitkara D. Topical application of vitamin D3-loaded hybrid nanosystem to offset imiquimod-induced psoriasis. AAPS PharmSciTech. 2021;22(7):238.
Jaiswal PK, Das S, Das MK. Boosting the skin delivery of curcumin through stearic acid-ethyl cellulose blend hybrid nanocarriers-based approach for mitigating psoriasis. Int J Appl Pharm. 2021;13(3):150–64.
Pukale SS, Sharma S, Dalela M, et al. Multi-component clobetasol-loaded monolithic lipid-polymer hybrid nanoparticles ameliorate imiquimod-induced psoriasis-like skin inflammation in Swiss albino mice. Acta Biomater. 2020;115:393–409.
Zhang S, Wang J, Liu L, et al. Efficacy and safety of curcumin in psoriasis: preclinical and clinical evidence and possible mechanisms. Front Pharmacol. 2022;13: 903160.
Anand P, Kunnumakkara AB, Newman RA, et al. Bioavailability of curcumin: problems and promises. Molecular Pharmaceutics. 2007;4(6):807–18.
Doppalapudi S, Jain A, Chopra DK, Khan W. Psoralen loaded liposomal nanocarriers for improved skin penetration and efficacy of topical PUVA in psoriasis. Eur J Pharm Sci. 2017;96:515–29.
Pitzanti G, Rosa A, Nieddu M, et al. Transcutol® P containing SLNs for improving 8-methoxypsoralen skin delivery. Pharmaceutics. 2020;12(10):973.
Akiba I, Terada N, Hashida S, et al. Encapsulation of a hydrophobic drug into a polymer-micelle core explored with synchrotron SAXS. Langmuir. 2010;26(10):7544–51.
Dave V, Tak K, Sohgaura A, et al. Lipid-polymer hybrid nanoparticles: synthesis strategies and biomedical applications. J Microbiol Methods. 2019;160:130–42.
Hu FQ, Wu XL, Du YZ, et al. Cellular uptake and cytotoxicity of shell crosslinked stearic acid-grafted chitosan oligosaccharide micelles encapsulating doxorubicin. Eur J Pharm Biopharm. 2008;69(1):117–25.
Yuan H, Lu LJ, Du YZ, et al. Stearic acid-g-chitosan polymeric micelle for oral drug delivery: in vitro transport and in vivo absorption. Mol Pharm. 2011;8(1):225–38.
Yu F, Tu Y, Luo S, et al. Dual-drug backboned polyprodrug with a predefined drug combination for synergistic chemotherapy. Nano Lett. 2021;21(5):2216–23.
Nava-Arzaluz MG, Piñón-Segundo E, Ganem-Rondero A. Single emulsion-solvent evaporation technique and modifications for the preparation of pharmaceutical polymeric nanoparticles. Recent Patent on Drug Delivery and Formulation. 2012;6(3):209–23.
Duan R, Li C, Wang F, et al. Polymer-lipid hybrid nanoparticles-based paclitaxel and etoposide combinations for the synergistic anticancer efficacy in osteosarcoma. Colloids Surf B. 2017;159:880–7.
Anjum MM, Kanoujia J, Parashar P, et al. Evaluation of a polymer-lipid-polymer system utilising hybrid nanoparticles of dapsone as a novel antiacne agent. Curr Drug Ther. 2016;11:86–100.
Huang Q, Cai T, Li Q, et al. Preparation of psoralen polymer-lipid hybrid nanoparticles and their reversal of multidrug resistance in MCF-7/ADR cells. Drug Deliv. 2018;25(1):1056–66.
Dave V, Yadav RB, Kushwaha K, et al. Lipid-polymer hybrid nanoparticles: development & statistical optimization of norfloxacin for topical drug delivery system. Bioact Mater. 2017;2(4):269–80.
Khan MA, Khan S, Kazi M, et al. Norfloxacin loaded lipid polymer hybrid nanoparticles for oral administration: fabrication, characterization, in silico modelling and toxicity evaluation. Pharmaceutics. 2021;13(10):1632.
Ling G, Zhang P, Zhang W, et al. Development of novel self-assembled DS-PLGA hybrid nanoparticles for improving oral bioavailability of vincristine sulfate by P-gp inhibition. J Control Release. 2010;148(2):241–9.
Dash S, Murthy PN, Nath LK, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67:217–23.
Madan JR, Khobaragade S, Dua K, Awasthi R. Formulation, optimization, and in vitro evaluation of nanostructured lipid carriers for topical delivery of Apremilast. Dermatol Ther. 2020;33(3): e13370.
Asad MI, Khan D, Rehman AU, et al. Development and in vitro/in vivo evaluation of pH-sensitive polymeric nanoparticles loaded hydrogel for the management of psoriasis. Nanomaterials (Basel). 2021;11(12):3433.
Sun L, Liu Z, Wang L, et al. Enhanced topical penetration, system exposure and anti-psoriasis activity of two particle-sized, curcumin-loaded PLGA nanoparticles in hydrogel. J Control Release. 2017;254:44–54.
Talele S, Nikam P, Gosh B, et al. A research article on nanogel as topical promising drug delivery for diclofenac sodium. Int J Pharm Ed Res. 2017;51(4S):S580–7.
Rajan R, Vasudevan DT. Effect of permeation enhancers on the penetration mechanism of transfersomal gel of ketoconazole. J Adv Pharm Technol Res. 2012;3(2):112–6.
Singha LR, Das MK. Effect of Mesua ferrea Linn. seed kernel oil on percutaneous absorption of diltiazem hydrochloride through pig ear epidermis: a mechanistic study. J Drug Deliv Sci Technol. 2021;64:102628.
Mei Z, Chen H, Weng T, et al. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur J Pharm Biopharm. 2003;56(2):189–96.
Tripathi P, Kumar A, Jain PK, Patel JR. Carbomer gel bearing methotrexate loaded lipid nanocontainers shows improved topical delivery intended for effective management of psoriasis. Int J Biol Macromol. 2018;120(Pt A):1322–34.
Kumar JR, Sulekha RK. Design and development of ciprofloxacin lipid polymer hybrid nanoparticle by response surface methodology. Res J Pharm Technol. 2020;13(7):3249–56.
Gangadevi V, Thatikonda S, Pooladanda V, et al. Selenium nanoparticles produce a beneficial effect in psoriasis by reducing epidermal hyperproliferation and inflammation. J Nanobiotechnol. 2021;19(1):101.
Gajra B, Dalwadi C, Patel R. Formulation and optimization of itraconazole polymeric lipid hybrid nanoparticles (Lipomer) using Box Behnken design. Daru J Pharm Sci. 2015;23(1):3.
Mukherjee A, Waters AK, Kalyan P, et al. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: state of the art, emerging technologies, and perspectives. Int J Nanomed. 2019;14:1937–52.
Sohaib M, Shah SU, Shah KU, et al. Physicochemical characterization of chitosan-decorated finasteride solid lipid nanoparticles for skin drug delivery. Biomed Res Int. 2022:7792180.
Mirnezami SMS, Heydarinasab A, Akbarzadehkhyavi A, et al. Development and optimization of lipid-polymer hybrid nanoparticles containing melphalan using central composite design and its effect on ovarian cancer cell lines. Iran J Pharm Res. 2021;20(4):213–228.
Sharma N, Madan P, Lin S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: a co-surfactant study. Asian J Pharm Sci. 2016;11(3):404–16.
Dali P, Shende P. Self-assembled lipid polymer hybrid nanoparticles using combinational drugs for migraine via intranasal route. AAPS PharmSciTech. 2022;24(1):20.
Imam SS, Gilani SJ, Bin Jumah MN, et al. Harnessing lipid polymer hybrid nanoparticles for enhanced oral bioavailability of thymoquinone: in vitro and in vivo assessments. Polymers (Basel). 2022;14(18):3705.
Ebrahimian M, Mahvelati F, Malaekeh-Nikouei B, et al. Bromelain loaded lipid-polymer hybrid nanoparticles for oral delivery: formulation and characterization. Appl Biochem Biotechnol. 2022;194(8):3733–48.
Shafie MA, Fayek H. Formulation and evaluation of betamethasone sodium phosphate loaded nanoparticles for ophthalmic delivery. J Clin Experiment Ophthalmol. 2013;4:1–11.
Hussain Z, Pandey M, Thu HE, et al. Hyaluronic acid functionalization improves dermal targeting of polymeric nanoparticles for management of burn wounds: in vitro, ex vivo and in vivo evaluations. Biomed Pharmacother. 2022;150: 112992.
Jangde R, Elhassan GO, Khute S, et al. Hesperidin-loaded lipid polymer hybrid nanoparticles for topical delivery of bioactive drugs. Pharmaceuticals. 2022;15(2):211.
Desfrançois C, Auzély R, Texier I. Lipid nanoparticles and their hydrogel composites for drug delivery: a review. Pharmaceuticals (Basel). 2018;11(4):118.
Tahir N, Madni A, Correia A, et al. Lipid-polymer hybrid nanoparticles for controlled delivery of hydrophilic and lipophilic doxorubicin for breast cancer therapy. Int J Nanomed. 2019;14:4961–74.
Yang P, Zhang L, Wang T, et al. Doxorubicin and edelfosine combo-loaded lipid-polymer hybrid nanoparticles for synergistic anticancer effect against drug-resistant osteosarcoma. Onco Targets and Therapy. 2020;13:8055–67.
El-Habashy SE, Allam AN, El-Kamel AH. Ethylcellulose nanoparticles as a platform to decrease ulcerogenic potential of piroxicam: formulation and in vitro/in vivo evaluation. Int J Nanomed. 2016;11:2369–80.
Kumar S, Randhawa JK. Solid lipid nanoparticles of stearic acid for the drug delivery of paliperidone. RSC Adv. 2015;5:68743–50.
Quirós-Fallas MI, Wilhelm-Romero K, Quesada-Mora S, et al. Curcumin hybrid lipid polymeric nanoparticles: antioxidant activity, immune cellular response, and cytotoxicity evaluation. Biomedicines. 2022;10(10):2431.
Laxmi RJ, Karthikeyan R, Babu PS, et al. Formulation and evaluation of antipsoriatic gel using natural excipients. Journal of Acute Disease. 2013;2(2):115–21.
Gupta U, Singh V, Kumar V, Khajuria Y. Spectroscopic studies of cholesterol: Fourier transform infra-red and vibrational frequency analysis. Material Focus. 2014;3:1–7.
Kaur K, Kumar P, Kush P. Amphotericin B loaded ethyl cellulose nanoparticles with magnified oral bioavailability for safe and effective treatment of fungal infection. Biomed Pharmacother. 2020;128: 110297.
Akhlaghi S, Rabbani S, Alavi S, et al. Green formulation of curcumin loaded lipid-based nanoparticles as a novel carrier for inhibition of post-angioplasty restenosis. Mater Sci Eng. C, Mater Biol Appl. 2019;105:110037.
Fang JY, Fang CL, Liu CH, et al. Lipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur J Pharm Biopharm. 2008;70(2):633–40.
Salehiabar M, Nosrati H, Javani E, et al. Production of biological nanoparticles from bovine serum albumin as controlled release carrier for curcumin delivery. Int J Biol Macromol. 2018;115:83–9.
Ishak RAH, Mostafa NM, Kamel AO. Stealth lipid polymer hybrid nanoparticles loaded with rutin for effective brain delivery-comparative study with the gold standard (Tween 80): optimization, characterization and biodistribution. Drug Delivery. 2017;24(1):1874–90.
Zhang L, Chan JM, Gu FX, et al. Self-assembled lipid–polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano. 2008;2(8):1696–702.
Lukić M, Pantelić I, Savić SD. Towards optimal pH of the skin and topical formulations: from the current state of the art to tailored products. Cosmetics. 2021;8(3):69.
Kaur N, Sharma K, Bedi N. Topical nanostructured lipid carrier based hydrogel of mometasone furoate for the treatment of psoriasis. Pharm Nanotechnol. 2018;6(2):133–43.
Parvez S, Yadagiri G, Gedda MR, et al. Modified solid lipid nanoparticles encapsulated with Amphotericin B and Paromomycin: an effective oral combination against experimental murine visceral leishmaniasis. Sci Rep. 2020;10:12243.
Singh R, Lillard JW Jr. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–23.
Singh R, Koppu S, Perche PO, Feldman SR. The cytokine mediated molecular pathophysiology of psoriasis and its clinical implications. Int J Mol Sci. 2021;22(23):12793.
Woo YR, Cho DH, Park HJ. Molecular mechanisms and management of a cutaneous inflammatory disorder: psoriasis. Int J Mol Sci. 2017;18(12):2684.
Panonnummal R, Jayakumar R, Sabitha M. Comparative anti-psoriatic efficacy studies of clobetasol loaded chitin nanogel and marketed cream. Eur J Pharm Sci. 2017;96:193–206.
Shakeel F, Baboota S, Ahuja A, et al. Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J Nanobiotechnol. 2008;9(6):8.
Vashisth I, Ahad A, Aqil M, Agarwal SP. Investigating the potential of essential oils as penetration enhancer for transdermal losartan delivery: effectiveness and mechanism of action. Asian J Pharm Sci. 2014;9(5):260–7.
van der Fits L, Mourits S, Voerman JS, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–45.
Wu L, Liu G, Wang W, et al. Cyclodextrin-modified CeO2 nanoparticles as a multifunctional nanozyme for combinational therapy of psoriasis. Int J Nanomed. 2020;15:2515–27.
Fereig SA, El-Zaafarany GM, Arafa MG, et al. Tacrolimus-loaded chitosan nanoparticles for enhanced skin deposition and management of plaque psoriasis. Carbohyd Polym. 2021;268: 118238.
Mao KL, Fan ZL, Yuan JD, et al. Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids Surf, B. 2017;160:704–14.
Jain S, Mittal A, Jain KA. Enhanced topical delivery of cyclosporin-A using PLGA nanoparticles as carrier. Curr Nanosci. 2011;7(4):524–30.
De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(s1):46–8.
Acknowledgements
The authors are thankful and grateful to the Drug Delivery Research Laboratory (DDRL), Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh, Assam, India, for providing the platform to conduct and carry out this research work.
Funding
This work was financially supported by All India Council for Technical Education (AICTE)-PG Scholarship Scheme.
Author information
Authors and Affiliations
Contributions
All authors have contributed equally and approved the final version of the manuscript as submitted. TJ: experimental works, data processing, interpretation of data, statistical analysis, and manuscript writing; SD: figure design, statistical analysis, and interpretation; MKD: conception of the study, conceived, designed, and contributed to the formal analysis of the study, and reviewed/edited the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The Institutional Animal Ethical Committee (IAEC) (Regd. No.1576/GO/Re/S/11/CPCSEA Dated:30/10/2018) of Dibrugarh University, Dibrugarh, Assam, India, approved the experimental protocol with approval number IAEC/DU/209, dated:26/2/2022. All the animal experiments were conducted following the guidelines by the Institutional Animal Ethics Committee (IAEC) of Dibrugarh University, Dibrugarh, Assam, India (Approval No. IAEC/DU/209, Dated. 26/02/2022).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jamatia, T., Das, S. & Das, M.K. Topical Delivery of Methoxsalen Co-loaded Curcumin Using Hybrid Nanocarrier-Based Polymeric Hydrogel for Synergistic Therapy in the Treatment of Psoriasis. J Pharm Innov 18, 2305–2324 (2023). https://doi.org/10.1007/s12247-023-09794-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09794-7